- Joined
- Jun 27, 2018
- Messages
- 31,528
- Points
- 113
Here’s How the AstraZeneca COVID-19 Vaccine Compares to Pfizer’s and Moderna’s
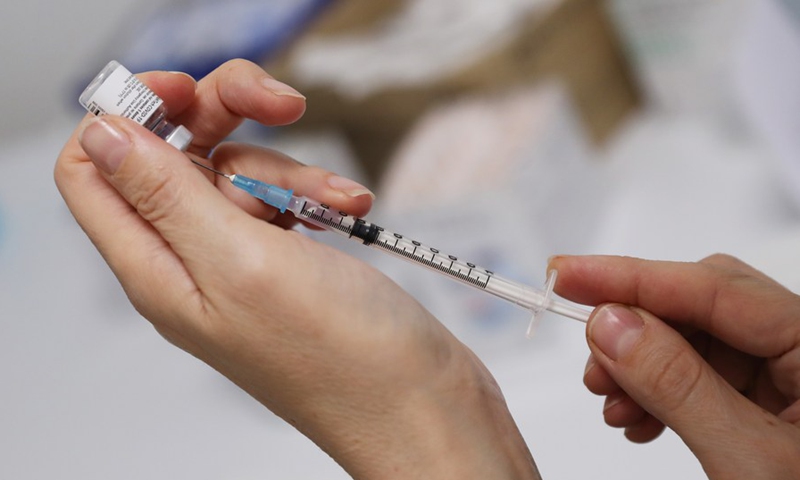
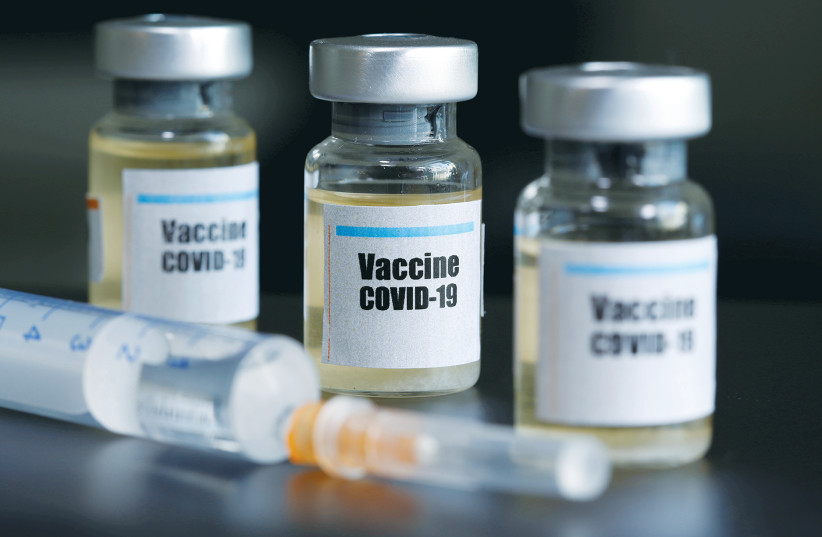
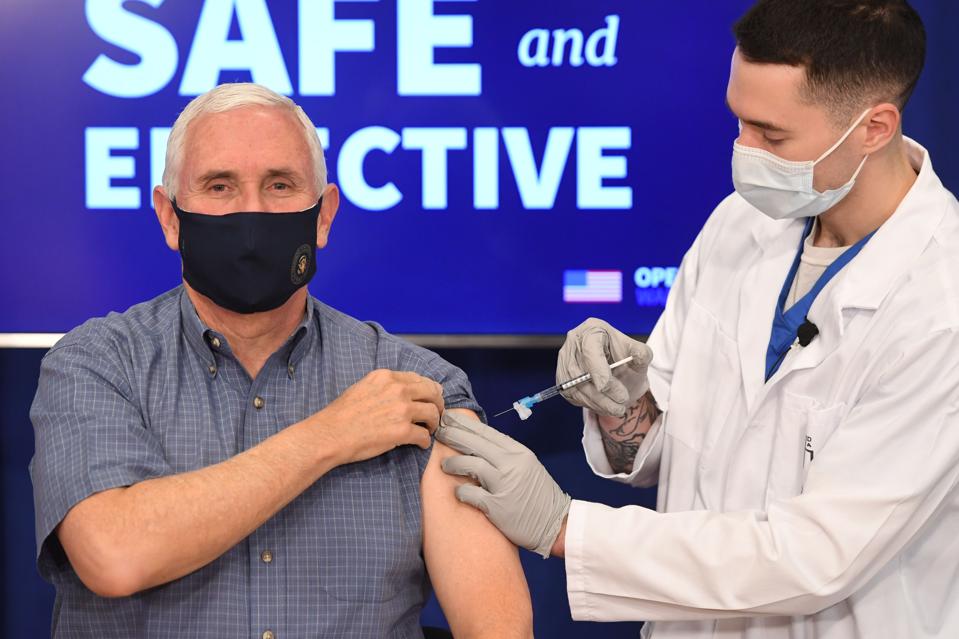
AstraZeneca’s COVID-19 vaccine has not yet been submitted for U.S. approval.
Research from the University of Oxford and AstraZeneca shows that a single shot of the two-step vaccine slows transmission—and that delaying the second dose might be beneficial.
South Africa has stopped offering the AstraZeneca-Oxford vaccine to its citizens following reports that it offers only little protection against the country’s dominant coronavirus variant.
The United Kingdom became the first country to approve AstraZeneca’s COVID-19 vaccine for emergency use on Dec. 30, just weeks after Pfizer’s and Moderna’s respective vaccines received a green light from the Food and Drug Administration in the United States. The approval is another promising sign in the global immunization rollout—especially because this option, developed by the University of Oxford and biopharmaceutical company AstraZeneca, could be key to reaching people in rural and underfunded areas.
Advertisement - Continue Reading Below
Unlike its competitors, the AstraZeneca COVID-19 vaccine can be stored at higher temperatures, costs less per dose, and uses different technology to immunize people. New findings out of South Africa, however, could interrupt the vaccine’s path to approval: It appears to offer only minimal protection against the country’s dominant, more contagious variant, which is rapidly spreading worldwide.
Although vaccine hasn’t been approved for use in the U.S. yet, here’s what we know about it so far, and how it stacks up against Pfizer’s and Moderna’s.
Advertisement - Continue Reading Below
How does the AstraZeneca COVID-19 vaccine work?
AstraZeneca’s vaccine uses adenovirus-vectored technology. Translation: It’s a harmless, modified version of a common cold virus that usually only spreads among chimpanzees. This altered virus can’t make you sick, but it carries a gene from the novel coronavirus’ spike protein, the portion of the virus that triggers an immune response. This allows the immune system to manufacture antibodies that work against COVID-19, teaching your body how to respond should you become infected.
In other words, AstraZeneca’s vaccine mimics a COVID-19 infection without its life-threatening side effects, per a release from the company. The reason researchers chose a chimpanzee adenovirus is simple: The modified virus needs to be new to the people being vaccinated—otherwise, the body won’t create those all-important antibodies. Anyone could already have antibodies for a cold spread among humans, but far fewer people have been exposed to a cold spread among chimps.
Advertisement - Continue Reading Below
Initially, the vaccine was administered with two shots spaced about a month apart. But new research from Oxford and AstraZeneca appears to show that the vaccine’s efficacy actually goes up when the second dose is delivered more than 12 weeks after the first. (More on this study’s findings below.)
The Pfizer-BioNTech and Moderna vaccines, meanwhile, rely on mRNA technology, which essentially introduces a piece of genetic code that tricks the body into producing COVID-19 antibodies, no virus required. Both approved vaccines require two shots spaced about a month apart. Although no adenovirus-vectored vaccine has been approved for human use before, companies like Johnson & Johnson, CanSino, and NantKwest are all working on their own versions.
Advertisement - Continue Reading Below
How does the AstraZeneca vaccine compare to the Moderna and Pfizer vaccines?
Storage and distribution
AstraZeneca’s vaccine is the easiest to transport so far—it can be stored for up to six months between 36 and 46°F, normal refrigerator temperatures. The Moderna and Pfizer options, meanwhile, must be stored at subzero temperatures until they’re ready to be used, at -4°F and -94°F, respectively. (mRNA technology is relatively fragile compared to adenovirus-vectored tech, meaning it must be kept at much lower temperatures to remain effective and stable.)
AstraZeneca’s higher storage temperature could make distribution much easier. “A clinic, a nursing home, or even [regional] health departments may not have freezers that can hold things at -94°F,” says Kawsar Talaat, M.D., an infectious disease doctor, vaccine researcher, and assistant professor in the department of International Health at Johns Hopkins University. Being able to use a typical fridge “allows time for distribution, allows the vaccine time to get to more rural areas, [and allows vaccines] to be kept at a clinic for a longer period of time.”
Advertisement - Continue Reading Below
Cost
The new vaccine also beats its competitors on price: AstraZeneca’s vaccine costs providers about $4 per dose, while Pfizer’s costs $20 and Moderna’s costs $33, Al Jazeera reports. These prices will most likely fluctuate as time goes on and the vaccines evolve.
Side effects
All three vaccines’ side effects are similar, including potential injection site pain and flu-like symptoms, including fever, fatigue, headaches, and muscle pain, which are to be expected as your immune system is primed, especially after the second dose.
Advertisement - Continue Reading Below
Efficacy
The two mRNA vaccines have a slight edge in efficacy; both Pfizer and Moderna report being about 95% effective against COVID-19 after the second shot in clinical trials. (For comparison, the annual flu shot is usually between 40 and 60% effective, per the CDC.) They also appear to reduce the risk of severe illness even if you do become infected with SARS-CoV-2.
The new AstraZeneca study, which has not yet been peer-reviewed, found the vaccine is 76% effective against the current, dominant strain of the novel coronavirus for up to three months after just one dose. Remarkably, it also appears to show that the vaccine becomes more effective with a longer wait between doses; infections were less likely among those who received their booster more than 12 weeks after their initial shot compared to those who received their booster less than six weeks after. More research is needed to confirm the significance of these findings, however.
Advertisement - Continue Reading Below
prevention premium button
If the data checks out, this could free up reserves of second-dose AstraZeneca vaccines to be used as first doses, potentially bolstering the global immunization effort. Since each vaccine is unique, though, separate trials recommending a longer wait between doses of the Pfizer and Moderna vaccines will be necessary.
Advertisement - Continue Reading Below
Every available vaccine, along with Johnson & Johnson’s (which also has yet to be approved in the U.S.), appears to offer lower protection against the new, more infectious COVID-19 variants currently spreading in the U.S. and abroad. New research from South Africa on the AstraZeneca vaccine found an under-25% efficacy against mild and moderate illness from the country’s more contagious B.1.351 variant, failing to meet the threshold for emergency approval. As a result, South Africa has since stopped offering the AstraZeneca vaccine to its citizens.
For now, the best way to avoid the variants is to continue following disease prevention guidelines and to get an authorized vaccine as soon as you’re eligible.
Advertisement - Continue Reading Below
Which COVID-19 vaccine is the best?
There’s no “best” vaccine option, as there’s not enough research to confirm that yet. Vaccines aren’t a silver bullet, especially as the pandemic rages on: They must be combined with masks, hand-washing, and social distancing to work as effectively as possible, per the CDC. No matter which COVID-19 vaccine becomes available to you first, you can feel confident in its ability to protect you, as long as you continue being cautious until positive cases, hospitalizations, and deaths are significantly reduced nationwide.
In the meantime, it’s likely “that all the manufacturers are working on making their vaccines more stable at easier-to-manage temperatures,” Dr. Talaat explains. Similarly, manufacturers are likely working toward greater protection against new variants. As their formulations change, their pros and cons will, too.
Advertisement - Continue Reading Below
For now, we can be thankful that AstraZeneca’s vaccine is being studied more. “The next generation of vaccines, like AstraZeneca’s, which is kept at refrigerator temperatures, is a major advancement,” Dr. Talaat says. “When you’re talking about distribution to the entire world, it’s much easier to do because we already keep vaccines cold. It’s a lot harder to keep things frozen.”
This article is accurate as of press time. However, as the COVID-19 pandemic rapidly evolves and the scientific community’s understanding of the novel coronavirus develops, some of the information may have changed since it was last updated. While we aim to keep all of our stories up to date, please visit online resources provided by the CDC, WHO, and your local public health department to stay informed on the latest news. Always talk to your doctor for professional medical advice.
Go here to join Prevention Premium (our best value, all-access plan), subscribe to the magazine, or get digital-only access.
This content is created and maintained by a third party, and imported onto this page to help users provide their email addresses. You may be able to find more information about this and similar content at piano.io
More From
AstraZeneca’s COVID-19 vaccine has not yet been submitted for U.S. approval.
Research from the University of Oxford and AstraZeneca shows that a single shot of the two-step vaccine slows transmission—and that delaying the second dose might be beneficial.
South Africa has stopped offering the AstraZeneca-Oxford vaccine to its citizens following reports that it offers only little protection against the country’s dominant coronavirus variant.
The United Kingdom became the first country to approve AstraZeneca’s COVID-19 vaccine for emergency use on Dec. 30, just weeks after Pfizer’s and Moderna’s respective vaccines received a green light from the Food and Drug Administration in the United States. The approval is another promising sign in the global immunization rollout—especially because this option, developed by the University of Oxford and biopharmaceutical company AstraZeneca, could be key to reaching people in rural and underfunded areas.
Advertisement - Continue Reading Below
Unlike its competitors, the AstraZeneca COVID-19 vaccine can be stored at higher temperatures, costs less per dose, and uses different technology to immunize people. New findings out of South Africa, however, could interrupt the vaccine’s path to approval: It appears to offer only minimal protection against the country’s dominant, more contagious variant, which is rapidly spreading worldwide.
Although vaccine hasn’t been approved for use in the U.S. yet, here’s what we know about it so far, and how it stacks up against Pfizer’s and Moderna’s.
Advertisement - Continue Reading Below
How does the AstraZeneca COVID-19 vaccine work?
AstraZeneca’s vaccine uses adenovirus-vectored technology. Translation: It’s a harmless, modified version of a common cold virus that usually only spreads among chimpanzees. This altered virus can’t make you sick, but it carries a gene from the novel coronavirus’ spike protein, the portion of the virus that triggers an immune response. This allows the immune system to manufacture antibodies that work against COVID-19, teaching your body how to respond should you become infected.
In other words, AstraZeneca’s vaccine mimics a COVID-19 infection without its life-threatening side effects, per a release from the company. The reason researchers chose a chimpanzee adenovirus is simple: The modified virus needs to be new to the people being vaccinated—otherwise, the body won’t create those all-important antibodies. Anyone could already have antibodies for a cold spread among humans, but far fewer people have been exposed to a cold spread among chimps.
Advertisement - Continue Reading Below
Initially, the vaccine was administered with two shots spaced about a month apart. But new research from Oxford and AstraZeneca appears to show that the vaccine’s efficacy actually goes up when the second dose is delivered more than 12 weeks after the first. (More on this study’s findings below.)
The Pfizer-BioNTech and Moderna vaccines, meanwhile, rely on mRNA technology, which essentially introduces a piece of genetic code that tricks the body into producing COVID-19 antibodies, no virus required. Both approved vaccines require two shots spaced about a month apart. Although no adenovirus-vectored vaccine has been approved for human use before, companies like Johnson & Johnson, CanSino, and NantKwest are all working on their own versions.
Advertisement - Continue Reading Below
How does the AstraZeneca vaccine compare to the Moderna and Pfizer vaccines?
Storage and distribution
AstraZeneca’s vaccine is the easiest to transport so far—it can be stored for up to six months between 36 and 46°F, normal refrigerator temperatures. The Moderna and Pfizer options, meanwhile, must be stored at subzero temperatures until they’re ready to be used, at -4°F and -94°F, respectively. (mRNA technology is relatively fragile compared to adenovirus-vectored tech, meaning it must be kept at much lower temperatures to remain effective and stable.)
AstraZeneca’s higher storage temperature could make distribution much easier. “A clinic, a nursing home, or even [regional] health departments may not have freezers that can hold things at -94°F,” says Kawsar Talaat, M.D., an infectious disease doctor, vaccine researcher, and assistant professor in the department of International Health at Johns Hopkins University. Being able to use a typical fridge “allows time for distribution, allows the vaccine time to get to more rural areas, [and allows vaccines] to be kept at a clinic for a longer period of time.”
Advertisement - Continue Reading Below
Cost
The new vaccine also beats its competitors on price: AstraZeneca’s vaccine costs providers about $4 per dose, while Pfizer’s costs $20 and Moderna’s costs $33, Al Jazeera reports. These prices will most likely fluctuate as time goes on and the vaccines evolve.
Side effects
All three vaccines’ side effects are similar, including potential injection site pain and flu-like symptoms, including fever, fatigue, headaches, and muscle pain, which are to be expected as your immune system is primed, especially after the second dose.
Advertisement - Continue Reading Below
Efficacy
The two mRNA vaccines have a slight edge in efficacy; both Pfizer and Moderna report being about 95% effective against COVID-19 after the second shot in clinical trials. (For comparison, the annual flu shot is usually between 40 and 60% effective, per the CDC.) They also appear to reduce the risk of severe illness even if you do become infected with SARS-CoV-2.
The new AstraZeneca study, which has not yet been peer-reviewed, found the vaccine is 76% effective against the current, dominant strain of the novel coronavirus for up to three months after just one dose. Remarkably, it also appears to show that the vaccine becomes more effective with a longer wait between doses; infections were less likely among those who received their booster more than 12 weeks after their initial shot compared to those who received their booster less than six weeks after. More research is needed to confirm the significance of these findings, however.
Advertisement - Continue Reading Below
prevention premium button
If the data checks out, this could free up reserves of second-dose AstraZeneca vaccines to be used as first doses, potentially bolstering the global immunization effort. Since each vaccine is unique, though, separate trials recommending a longer wait between doses of the Pfizer and Moderna vaccines will be necessary.
Advertisement - Continue Reading Below
Every available vaccine, along with Johnson & Johnson’s (which also has yet to be approved in the U.S.), appears to offer lower protection against the new, more infectious COVID-19 variants currently spreading in the U.S. and abroad. New research from South Africa on the AstraZeneca vaccine found an under-25% efficacy against mild and moderate illness from the country’s more contagious B.1.351 variant, failing to meet the threshold for emergency approval. As a result, South Africa has since stopped offering the AstraZeneca vaccine to its citizens.
For now, the best way to avoid the variants is to continue following disease prevention guidelines and to get an authorized vaccine as soon as you’re eligible.
Advertisement - Continue Reading Below
Which COVID-19 vaccine is the best?
There’s no “best” vaccine option, as there’s not enough research to confirm that yet. Vaccines aren’t a silver bullet, especially as the pandemic rages on: They must be combined with masks, hand-washing, and social distancing to work as effectively as possible, per the CDC. No matter which COVID-19 vaccine becomes available to you first, you can feel confident in its ability to protect you, as long as you continue being cautious until positive cases, hospitalizations, and deaths are significantly reduced nationwide.
In the meantime, it’s likely “that all the manufacturers are working on making their vaccines more stable at easier-to-manage temperatures,” Dr. Talaat explains. Similarly, manufacturers are likely working toward greater protection against new variants. As their formulations change, their pros and cons will, too.
Advertisement - Continue Reading Below
For now, we can be thankful that AstraZeneca’s vaccine is being studied more. “The next generation of vaccines, like AstraZeneca’s, which is kept at refrigerator temperatures, is a major advancement,” Dr. Talaat says. “When you’re talking about distribution to the entire world, it’s much easier to do because we already keep vaccines cold. It’s a lot harder to keep things frozen.”
This article is accurate as of press time. However, as the COVID-19 pandemic rapidly evolves and the scientific community’s understanding of the novel coronavirus develops, some of the information may have changed since it was last updated. While we aim to keep all of our stories up to date, please visit online resources provided by the CDC, WHO, and your local public health department to stay informed on the latest news. Always talk to your doctor for professional medical advice.
Go here to join Prevention Premium (our best value, all-access plan), subscribe to the magazine, or get digital-only access.
This content is created and maintained by a third party, and imported onto this page to help users provide their email addresses. You may be able to find more information about this and similar content at piano.io
More From