- Joined
- Aug 7, 2008
- Messages
- 1,204
- Points
- 48
https://tw.news.yahoo.com/降血壓藥驚傳含致癌物-這2款藥品下架回收3188萬顆-034228681.html
降血壓藥驚傳含致癌物!這2款藥品下架回收3188萬顆

新頭殼
4.5k 人追蹤
新頭殼newtalk |張嘉哲 綜合報導
2019年3月23日 上午11:42

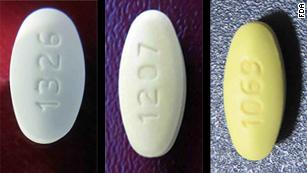
壓膜衣錠50毫克 (上)、壓寧悅膜衣錠50/12.5毫克 (下) 。 圖:食藥署/提供
[新頭殼newtalk] 衛福部食藥署日前接獲美國食藥局通知,印度一家原料藥廠生產的ARB類降血壓藥,含有致癌物「N-亞硝基-N-甲基-4-氨基丁酸」(NMBA),國內有「緩壓膜衣錠50毫克」與「壓寧悅膜衣錠50/12.5毫克」2款藥,使用到該廠原料藥,食藥署立刻進行清查,結果發現這兩款藥均有NMBA,目前已要求藥廠下架回收3188萬顆藥品。
美國食藥局在3月初在印度製藥廠Hetero的降血壓藥主成分發現第3種致癌物NMBA,因此對全球發出警訊,而食藥署於3月2日表示,國內有「緩壓膜衣錠50毫克」與「壓寧悅膜衣錠50/12.5毫克」2款藥使用到該廠原料藥,宣布共計3188萬顆已出貨的藥品需預防性下架。
食藥署藥品組科長洪國登表示,食藥署於3月初清查時,已先要求2家藥廠當天對2款產品預防性下架,先存放在藥局或醫院的倉庫,今檢驗報告出爐,確定2款降血壓藥的原料藥中驗到不純物後,也再要求2藥廠於1個月內完成回收
洪國登說,因運送時間、統計數量、處理退換貨事宜,回收日期至4月22日止,若未在期限內下架回收完畢,將會依《藥事法》94條,針對廠商或業者處以2萬元以上到10萬元以下罰鍰。
Blood pressure lowering drugs contain carcinogens! The two drugs were collected and recovered 31.88 million
[new head shell]
New head shell
4.5k person tracking
New head shell newtalk | Zhang Jiazhe Comprehensive report
March 23, 2019, 11:42 am
Pressed film ingot 50 mg (top), pressed Ning Yue film ingot 50/12.5 mg (bottom). Figure: Food and Drug Administration / Provided
Pressed film ingot 50 mg (top), pressed Ning Yue film ingot 50/12.5 mg (bottom). Figure: Food and Drug Administration / Provided
[New head shell newtalk] Weifu Department of Food and Drug Administration recently received notification from the US Food and Drug Administration that ARB blood pressure lowering drugs produced by a raw material drug factory in India contain carcinogen "N-nitroso-N-methyl-4- "Aminobutyric acid" (NMBA), there are two drugs in the country: "Relief film ingot 50 mg" and "pressing Ning Yue film ingot 50/12.5 mg". The drug is used in the factory, and the Food and Drug Administration immediately conducts an inventory. The results showed that both drugs had NMBA, and the drug factory has been required to remove 31.88 million drugs.
The US Food and Drug Administration discovered the third carcinogen NMBA in the main component of the antihypertensive drug of Hetero, an Indian pharmaceutical factory in early March, and therefore issued a warning to the world. The Food and Drug Administration said on March 2 that there is a "slow-pressure film" in the country. Two tablets of "50 mg of ingots" and "50/12.5 mg of pressed Ningfu film ingots" were used in the drug, and a total of 31.88 million drugs shipped were required to be removed.
Hong Guodeng, head of the drug group of the Food and Drug Administration, said that when the Food and Drug Administration inspected it in early March, it had requested two pharmaceutical companies to prevent the two products from being placed on the pharmacy or hospital warehouse. After confirming the detection of impurities in the raw materials of the two blood pressure lowering drugs, the 2 pharmaceutical companies are required to complete the recycling within one month.
Hong Guodeng said that due to the delivery time, the number of statistics, and the handling of return and exchange, the collection date will be until April 22, and if it is not completed within the time limit, it will be directed to the manufacturer or the manufacturer according to Article 94 of the Pharmaceutical Affairs Law. It is fined between 20,000 yuan and 100,000 yuan.
降血壓藥驚傳含致癌物!這2款藥品下架回收3188萬顆

新頭殼
4.5k 人追蹤
新頭殼newtalk |張嘉哲 綜合報導
2019年3月23日 上午11:42

壓膜衣錠50毫克 (上)、壓寧悅膜衣錠50/12.5毫克 (下) 。 圖:食藥署/提供
[新頭殼newtalk] 衛福部食藥署日前接獲美國食藥局通知,印度一家原料藥廠生產的ARB類降血壓藥,含有致癌物「N-亞硝基-N-甲基-4-氨基丁酸」(NMBA),國內有「緩壓膜衣錠50毫克」與「壓寧悅膜衣錠50/12.5毫克」2款藥,使用到該廠原料藥,食藥署立刻進行清查,結果發現這兩款藥均有NMBA,目前已要求藥廠下架回收3188萬顆藥品。
美國食藥局在3月初在印度製藥廠Hetero的降血壓藥主成分發現第3種致癌物NMBA,因此對全球發出警訊,而食藥署於3月2日表示,國內有「緩壓膜衣錠50毫克」與「壓寧悅膜衣錠50/12.5毫克」2款藥使用到該廠原料藥,宣布共計3188萬顆已出貨的藥品需預防性下架。
食藥署藥品組科長洪國登表示,食藥署於3月初清查時,已先要求2家藥廠當天對2款產品預防性下架,先存放在藥局或醫院的倉庫,今檢驗報告出爐,確定2款降血壓藥的原料藥中驗到不純物後,也再要求2藥廠於1個月內完成回收
洪國登說,因運送時間、統計數量、處理退換貨事宜,回收日期至4月22日止,若未在期限內下架回收完畢,將會依《藥事法》94條,針對廠商或業者處以2萬元以上到10萬元以下罰鍰。
Blood pressure lowering drugs contain carcinogens! The two drugs were collected and recovered 31.88 million
[new head shell]
New head shell
4.5k person tracking
New head shell newtalk | Zhang Jiazhe Comprehensive report
March 23, 2019, 11:42 am
Pressed film ingot 50 mg (top), pressed Ning Yue film ingot 50/12.5 mg (bottom). Figure: Food and Drug Administration / Provided
Pressed film ingot 50 mg (top), pressed Ning Yue film ingot 50/12.5 mg (bottom). Figure: Food and Drug Administration / Provided
[New head shell newtalk] Weifu Department of Food and Drug Administration recently received notification from the US Food and Drug Administration that ARB blood pressure lowering drugs produced by a raw material drug factory in India contain carcinogen "N-nitroso-N-methyl-4- "Aminobutyric acid" (NMBA), there are two drugs in the country: "Relief film ingot 50 mg" and "pressing Ning Yue film ingot 50/12.5 mg". The drug is used in the factory, and the Food and Drug Administration immediately conducts an inventory. The results showed that both drugs had NMBA, and the drug factory has been required to remove 31.88 million drugs.
The US Food and Drug Administration discovered the third carcinogen NMBA in the main component of the antihypertensive drug of Hetero, an Indian pharmaceutical factory in early March, and therefore issued a warning to the world. The Food and Drug Administration said on March 2 that there is a "slow-pressure film" in the country. Two tablets of "50 mg of ingots" and "50/12.5 mg of pressed Ningfu film ingots" were used in the drug, and a total of 31.88 million drugs shipped were required to be removed.
Hong Guodeng, head of the drug group of the Food and Drug Administration, said that when the Food and Drug Administration inspected it in early March, it had requested two pharmaceutical companies to prevent the two products from being placed on the pharmacy or hospital warehouse. After confirming the detection of impurities in the raw materials of the two blood pressure lowering drugs, the 2 pharmaceutical companies are required to complete the recycling within one month.
Hong Guodeng said that due to the delivery time, the number of statistics, and the handling of return and exchange, the collection date will be until April 22, and if it is not completed within the time limit, it will be directed to the manufacturer or the manufacturer according to Article 94 of the Pharmaceutical Affairs Law. It is fined between 20,000 yuan and 100,000 yuan.