- Joined
- Jun 27, 2018
- Messages
- 31,517
- Points
- 113
Major setback to the AstraZeneca vaccine causing chaos and confusion
There has been a call for calm as GPs raise legal fears about the AstraZeneca vaccine.
More than 1.6 million Australians have received the COVID-19 jab but a major setback to the AstraZeneca vaccine is causing chaos and confusion in the community.
Australian Medical Association President Dr Omar Khorshid today called for calm about the nation's vaccine roll-out.
"Right now we've got GPs, doctors, we've got hospitals, governments, scrambling to work out what it means for them and how we reset the programs," Dr Khorshid said.
The Pfizer vaccine is the preferred jab for Australians under 50, after AstraZeneca was linked to rare blood clots.
AstraZeneca COVID-19 vaccine vials (Getty)
(Getty)
The Australian Technical Advisory Group on Immunisation (ATAGI) issued updated advice recommending that the AstraZeneca jab be reserved for those over the age of 50, due to a rare but serious side effect of a blood clotting disorder.
While the AstraZeneca vaccine isn't banned for an aged group, some doctors were concerned they could face legal action if their patients suffered serious side-effects.
"We have strong, clear indemnity protection against any side-effects of the vaccine for patients and doctors," Health Minister Greg Hunt said.
However, Shadow Health Minister Mark Butler claims doctors are feeling "left in the lurch by government making decisions on the run".
There is also confusion over the vaccine timeline. The federal government previously aimed for all Australians to have their first dose by the end of October this year.
While Prime Minister Scott Morrison won't be drawn on a new target date, Trade Minister Dan Tehan said the goal was "by the end of the year".
However, Mr Hunt was quick to step back Mr Tehan's comments, saying there has been no change to the timeline. He would not be drawn on a specific date, other than to say all Australians will be vaccinated "as early as possible".
The AstraZeneca vaccine has much less stringent storage criteria compared to the Pfizer shot, presenting further logistical challenges for the government. (Nine)
Leading Australian epidemiologist Professor Nancy Baxter said the Federal Government needs to "entirely rethink" its coronavirus vaccination strategy after the AstraZeneca health warnings.
"The vaccine program frankly was already pretty much in disarray," Professor Baxter told Weekend Today.
"Now that we have to wait for a large part of the population for the Pfizer to come, they will have to rethink the program entirely."
Professor Baxter said Australians under 50 could still elect to receive the AstraZeneca vaccine if they chose, noting the risk remained remote.
"It's uncommon - about 1 in 100,000 to one in 200,000 people - meaning that 99,999 people who get the AstraZeneca vaccine do not have a problem," she said.
Around one-quarter of those who do develop the blood clotting reaction will die.
Prime Minister Scott Morrison on Friday refused to outline a revised timeframe for when all Australinas will have received their COVID-19 vaccines. (Alex Ellinghausen/Sydney Morning Herald)
Professor Baxter noted that this was still lower than the risk profile for many other drugs which continue to be used in Australia.
"When we give any medication, there's always some risks of side-effects. That's accepted because of the benefit of the medication," she said.
"If you got penicillin for an infection, there's ten times the risk there would be a serious drug reaction with that drug (compared to the AstraZeneca vaccine)."
Professor Baxter said the age cut-off was in part based on a weighing up of the risks of the vaccine versus the risks of contracting coronavirus.
"Older people are at more risk of COVID-19, so the risk-benefit equation balances out better for them," she said.
"Also, there is some data that indicates age is a risk factor, so younger people are more likely to get it (blood clots) than older people."
Leading Australian epidemiologist Professor Nancy Baxter has told Weekend Today the government needs to rethink its vaccination strategy "entirely". (Weekend Today)
It's expected that more data will be uncovered over the coming months.
The Federal Government has already responded to the ATAGI recommendation by contracting a further 20 million doses of the Pfizer shot in addition to 20 million already secured - enough to vaccinate the bulk of the adult Australian population - but those additional doses won't arrive in Australia until at least October.
Professor Baxter, who is the head of the Melbourne School of Population and Global Health, said Australia should already have enough Pfizer doses to progress through Phase 1B of its vaccine rollout.
This includes vaccinating all Australians over the age of 70 and Indigenous Australians over 55 - all of whom can still receive AstraZeneca under current health advice - but also many younger Australians, including healthcare workers, emergency responders and those with underlying medical conditions that make them vulnerable.
However, logistical concerns around administering the Pfizer vaccine are likely to further delay Australia's already tardy vaccination progress.
Australia's vaccine rollout is broken down into phases. (Graphic: Tara Blancato)
More ultra-low temperature freezers will likely need to be secured and getting the vaccine to more remote locations could prove problematic.
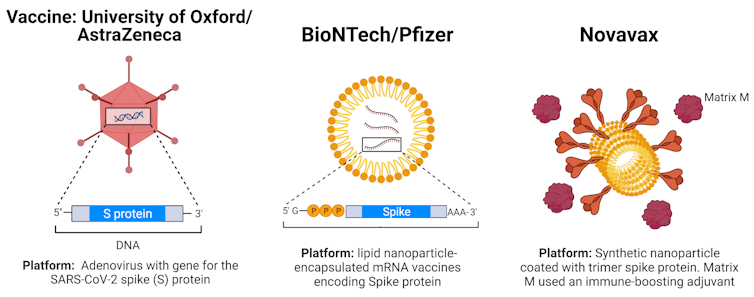
Australia's third vaccine option, Novavax, has been contracted for 51 million doses but is unlikely to be approved for use in Australia for several months as it is still undergoing phase three clinical trials in the United Kingdom.
"We have to reboot - they really need to rethink the program," Professor Baxter said.
"Once we get enough Pfizer to be vaccinating a large number of people, we need to look at how we approach it.
"We need to do it faster than we were hoping before, if we're hoping to get everyone vaccinated by the end of the year."
It's a goal that now appears unlikely, with Prime Minister Scott Morrison refusing to outline a revised timeline for the vaccination program at his National Cabinet update on Friday.
There has been a call for calm as GPs raise legal fears about the AstraZeneca vaccine.
More than 1.6 million Australians have received the COVID-19 jab but a major setback to the AstraZeneca vaccine is causing chaos and confusion in the community.
Australian Medical Association President Dr Omar Khorshid today called for calm about the nation's vaccine roll-out.
"Right now we've got GPs, doctors, we've got hospitals, governments, scrambling to work out what it means for them and how we reset the programs," Dr Khorshid said.
The Pfizer vaccine is the preferred jab for Australians under 50, after AstraZeneca was linked to rare blood clots.
AstraZeneca COVID-19 vaccine vials (Getty)
(Getty)
The Australian Technical Advisory Group on Immunisation (ATAGI) issued updated advice recommending that the AstraZeneca jab be reserved for those over the age of 50, due to a rare but serious side effect of a blood clotting disorder.
While the AstraZeneca vaccine isn't banned for an aged group, some doctors were concerned they could face legal action if their patients suffered serious side-effects.
"We have strong, clear indemnity protection against any side-effects of the vaccine for patients and doctors," Health Minister Greg Hunt said.
However, Shadow Health Minister Mark Butler claims doctors are feeling "left in the lurch by government making decisions on the run".
There is also confusion over the vaccine timeline. The federal government previously aimed for all Australians to have their first dose by the end of October this year.
While Prime Minister Scott Morrison won't be drawn on a new target date, Trade Minister Dan Tehan said the goal was "by the end of the year".
However, Mr Hunt was quick to step back Mr Tehan's comments, saying there has been no change to the timeline. He would not be drawn on a specific date, other than to say all Australians will be vaccinated "as early as possible".
The AstraZeneca vaccine has much less stringent storage criteria compared to the Pfizer shot, presenting further logistical challenges for the government. (Nine)
Leading Australian epidemiologist Professor Nancy Baxter said the Federal Government needs to "entirely rethink" its coronavirus vaccination strategy after the AstraZeneca health warnings.
"The vaccine program frankly was already pretty much in disarray," Professor Baxter told Weekend Today.
"Now that we have to wait for a large part of the population for the Pfizer to come, they will have to rethink the program entirely."
Professor Baxter said Australians under 50 could still elect to receive the AstraZeneca vaccine if they chose, noting the risk remained remote.
"It's uncommon - about 1 in 100,000 to one in 200,000 people - meaning that 99,999 people who get the AstraZeneca vaccine do not have a problem," she said.
Around one-quarter of those who do develop the blood clotting reaction will die.
Prime Minister Scott Morrison on Friday refused to outline a revised timeframe for when all Australinas will have received their COVID-19 vaccines. (Alex Ellinghausen/Sydney Morning Herald)
Professor Baxter noted that this was still lower than the risk profile for many other drugs which continue to be used in Australia.
"When we give any medication, there's always some risks of side-effects. That's accepted because of the benefit of the medication," she said.
"If you got penicillin for an infection, there's ten times the risk there would be a serious drug reaction with that drug (compared to the AstraZeneca vaccine)."
Professor Baxter said the age cut-off was in part based on a weighing up of the risks of the vaccine versus the risks of contracting coronavirus.
"Older people are at more risk of COVID-19, so the risk-benefit equation balances out better for them," she said.
"Also, there is some data that indicates age is a risk factor, so younger people are more likely to get it (blood clots) than older people."
Leading Australian epidemiologist Professor Nancy Baxter has told Weekend Today the government needs to rethink its vaccination strategy "entirely". (Weekend Today)
It's expected that more data will be uncovered over the coming months.
The Federal Government has already responded to the ATAGI recommendation by contracting a further 20 million doses of the Pfizer shot in addition to 20 million already secured - enough to vaccinate the bulk of the adult Australian population - but those additional doses won't arrive in Australia until at least October.
Professor Baxter, who is the head of the Melbourne School of Population and Global Health, said Australia should already have enough Pfizer doses to progress through Phase 1B of its vaccine rollout.
This includes vaccinating all Australians over the age of 70 and Indigenous Australians over 55 - all of whom can still receive AstraZeneca under current health advice - but also many younger Australians, including healthcare workers, emergency responders and those with underlying medical conditions that make them vulnerable.
However, logistical concerns around administering the Pfizer vaccine are likely to further delay Australia's already tardy vaccination progress.
Australia's vaccine rollout is broken down into phases. (Graphic: Tara Blancato)
More ultra-low temperature freezers will likely need to be secured and getting the vaccine to more remote locations could prove problematic.
Australia's third vaccine option, Novavax, has been contracted for 51 million doses but is unlikely to be approved for use in Australia for several months as it is still undergoing phase three clinical trials in the United Kingdom.
"We have to reboot - they really need to rethink the program," Professor Baxter said.
"Once we get enough Pfizer to be vaccinating a large number of people, we need to look at how we approach it.
"We need to do it faster than we were hoping before, if we're hoping to get everyone vaccinated by the end of the year."
It's a goal that now appears unlikely, with Prime Minister Scott Morrison refusing to outline a revised timeline for the vaccination program at his National Cabinet update on Friday.