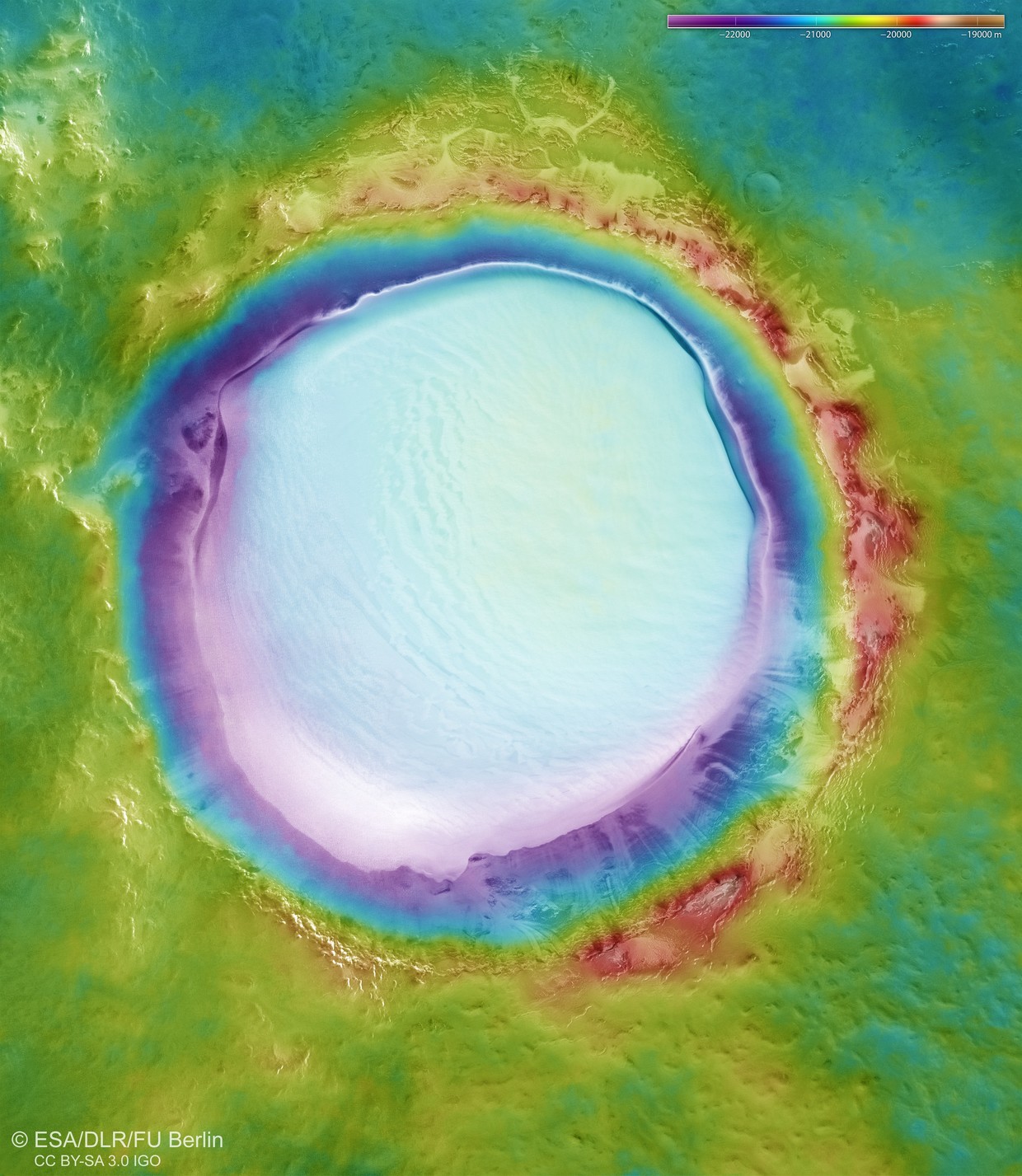
is this for real?theres water on mars?
May not be the H²O water even, as we know there is a non-drinkable version of HEAVY WATER used in Nuclear Power Plant called D²O. That tiny amount is nothing to be excited. Can not even sustain a AMK GRC for 1 month.
https://en.wikipedia.org/wiki/Semiheavy_water
Semiheavy water
From Wikipedia, the free encyclopedia
Jump to navigation Jump to search
Deuterium hydrogen oxide
 Names IUPAC name
Names IUPAC name
(O-2H1)Water
Other names
Deuterium hydrogen monoxide
Deuterium hydrogen oxide, Water-
d1 , Water-
d
Identifiers
3D model (
JSmol)
ChEBI
ChemSpider
InChI[show]
SMILES[show]
Properties
Chemical formula
H2HO (also HDO)
Molar mass 19.0214 g mol−1 Appearance Very pale blue, transparent liquid, very similar to regular water
Density 1.054 g cm−3
Melting point 3.81 °C (38.86 °F; 276.96 K)
Boiling point 101.42 °C (214.56 °F; 374.57 K)
Solubility in water
Reacts
log P −0.65
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
verify (
what is ?)
Infobox references
Semiheavy water is the result of replacing one of the
protium in
light water to
deuterium. It exists whenever there is water with light hydrogen (
protium, 1H) and deuterium (D or 2H) in the mix. This is because hydrogen atoms (hydrogen-1 and deuterium) are rapidly exchanged between water molecules. Water containing 50% H and 50% D in its hydrogen contains about 50% HDO and 25% each of H2O and D2O, in
dynamic equilibrium. In r
egular water, about 1 molecule in 3,200 is HDO (one hydrogen in 6,400 is D). By comparison,
heavy water D2O occurs at a proportion of about 1 molecule in 41 million (i.e., one in 6,4002). This makes semiheavy water far more common than "normal" heavy water.
The freezing point of semiheavy water is close to the freezing point of heavy water. (3.8°C)
https://en.wikipedia.org/wiki/Heavy_water
Heavy water
From Wikipedia, the free encyclopedia
Jump to navigation Jump to search
Not to be confused with
hard water or
tritiated water.

This article
needs additional citations for verification. Please help
improve this article by
adding citations to reliable sources. Unsourced material may be challenged and removed.
(March 2018) (Learn how and when to remove this template message)
Heavy water
 Names IUPAC name
Names IUPAC name
(2H2)Water
[3]
Other names
- Deuterium oxide[1]
- Water-d2[2]
- Dideuterium monoxide
Properties
Chemical formula
D
2O
Molar mass 20.0276 g mol−1 Appearance Colorless liquid
Odor Odorless
Density 1.107 g mL−1
Melting point 3.82 °C; 38.88 °F; 276.97 K
Boiling point 101.4 °C (214.5 °F; 374.5 K)
Solubility in water
Miscible
log P −1.38
Refractive index (
nD)
1.328
Viscosity 1.25 mPa s (at 20 °C)
Dipole moment
1.87 D
Hazards NFPA 704
0
1
0
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
verify (
what is ?)
Infobox references
Heavy water (
deuterium oxide,
2
H
2O,
D
2O) is a form of
water that contains a larger than normal amount of the
hydrogen isotope deuterium (2
H or D, also known as
heavy hydrogen), rather than the common
hydrogen-1 isotope (1
H or H, also called
protium) that makes up most of the hydrogen in normal water.
[4] The presence of deuterium gives the chemical different nuclear properties, and the increase of mass gives it different physical and chemical properties compared to normal "light water".
Contents
Explanation
Deuterium is a hydrogen isotope with a nucleus containing a
neutron and a
proton; the nucleus of a protium (normal hydrogen) atom consists of just a proton. The additional neutron makes a deuterium atom roughly twice as heavy as a protium atom.
A molecule of heavy water has two deuterium atoms in place of the two protium atoms of ordinary "light" water. The weight of a heavy water molecule, however, is not substantially different from that of a normal water molecule, because about 89% of the molecular weight of water comes from the single
oxygen atom rather than the two hydrogen atoms. The colloquial term
heavy water refers to a highly enriched water mixture that contains mostly deuterium oxide D
2O, but also some hydrogen-deuterium oxide (HDO) and a smaller number of ordinary hydrogen oxide H
2O molecules. For instance, the heavy water used in
CANDU reactors is 99.75% enriched by hydrogen atom-fraction—meaning that 99.75% of the hydrogen atoms are of the heavy type. For comparison,
ordinary water (the "ordinary water" used for a deuterium standard) contains only about 156 deuterium atoms per million hydrogen atoms, meaning that 0.0156% of the hydrogen atoms are of the heavy type.
Heavy water is not
radioactive. In its pure form, it has a density about 11% greater than water, but is otherwise physically and chemically similar. Nevertheless, the various differences in deuterium-containing water (especially affecting the biological properties) are larger than in any other commonly occurring
isotope-substituted compound because deuterium is unique among heavy
stable isotopes in being twice as heavy as the lightest isotope. This difference increases the
strength of water's hydrogen-oxygen bonds, and this in turn is enough to cause differences that are important to some biochemical reactions. The human body naturally contains deuterium equivalent to about five grams of heavy water, which is harmless. When a large fraction of water (> 50%) in higher organisms is replaced by heavy water, the result is
cell dysfunction and death.
[5]
Heavy water was first produced in 1932, a few months after the discovery of deuterium.
[6] With the discovery of
nuclear fission in late 1938, and the need for a
neutron moderator that captured few neutrons, heavy water became a component of early
nuclear energy research. Since then, heavy water has been an essential component in some types of reactors, both those that generate power and those designed to produce isotopes for nuclear weapons. These
heavy water reactors have the advantage of being able to run on natural uranium without using
graphite moderators that pose radiological
[7] and
dust explosion[8] hazards in the decommissioning phase. Most modern reactors use
enriched uranium with ordinary water as the moderator.
Other heavy forms of water
Semiheavy water
Semiheavy water, HDO, exists whenever there is water with light hydrogen (protium, 1
H) and deuterium (D or 2
H) in the mix. This is because hydrogen atoms (hydrogen-1 and deuterium) are rapidly exchanged between water molecules. Water containing 50% H and 50% D in its hydrogen actually contains about 50% HDO and 25% each of H
2O and D
2O, in
dynamic equilibrium. In normal water, about 1 molecule in 3,200 is HDO (one hydrogen in 6,400 is in the form of D), and heavy water molecules (D
2O) only occur in a proportion of about 1 molecule in 41 million (i.e. one in 6,4002). Thus
semiheavy water molecules are far more common than "pure" (homoisotopic) heavy water molecules.
Heavy-oxygen water
Water enriched in the heavier oxygen isotopes
17
O and
18
O is also commercially available, e.g., for
use as a non-radioactive isotopic tracer. It is "heavy water" as it is denser than normal water (H
218
O is approximately as dense as D
2O, H
217
O is about halfway between H
2O and D
2O)—but is rarely called heavy water, since it does not contain the deuterium that gives D2O its unusual nuclear and biological properties. It is more expensive than D2O due to the more difficult separation of 17O and 18O.
[9] H218O is also used for production of
fluorine-18 for
radiopharmaceuticals and
radiotracers and for
positron emission tomography.
Tritiated water
Tritiated water contains
tritium (3H) in place of protium (1H) or deuterium (2H), and therefore it is radioactive.
Physical properties
Physical properties of isotopologues of water
[10]
Property D2O (Heavy water) HDO (Semiheavy water) H2O (Light water) Freezing point 3.82 °C (38.88 °F) (276.97 K) 2.04 °C (35.67 °F) (275.19 K) 0.0 °C (32 °F) (273.15 K)
Boiling point 101.4 °C (214.5 °F) (374.55 K) 100.7 °C (213.3 °F) (373.85 K) 100.0 °C (212 °F) (373.15 K)
Density at
STP (g/
mL) 1.1056 1.054 0.9982 Temp. of maximum density 11.6 °C Unverified 3.98 °C
[11] Dynamic viscosity (at 20 °C,
mPa·
s) 1.2467 1.1248 1.0016
Surface tension (at 25 °C,
N/
m) 0.07187 0.07193 0.07198
Heat of fusion (
kJ/
mol) 6.132 6.227 6.00678
Heat of vaporisation (kJ/mol) 41.521 Unverified 40.657
pH (at 25 °C)
[12] 7.44 ("pD") 7.266 ("pHD") 7.0
pKb (at 25 °C)
[12] 7.44 ("p
Kb D2O") Unverified 7.0
Refractive index (at 20 °C, 0.5893
μm)
[13] 1.32844 Unverified 1.33335
The physical properties of water and heavy water differ in several respects. Heavy water is less dissociated than light water at given temperature, and the true concentration of D+ ions is less than
H+ ions would be for a light water sample at the same temperature. The same is true of OD− vs.
OH− ions. For heavy water Kw D2O (25.0 °C) = 1.35 × 10−15, and [D+ ] must equal [OD− ] for neutral water. Thus pKw D2O = p[OD−] + p[D+] = 7.44 + 7.44 = 14.87 (25.0 °C), and the p[D+] of neutral heavy water at 25.0 °C is 7.44.
The pD of heavy water is generally measured using pH electrodes giving a pH (apparent) value, or pHa, and at various temperatures a true acidic pD can be estimated from the directly pH meter measured pHa, such that pD+ = pHa (apparent reading from pH meter) + 0.41. The electrode correction for alkaline conditions is 0.456 for heavy water. The alkaline correction is then pD+ = pHa(apparent reading from pH meter) + 0.456. These corrections are slightly different from the differences in p[D+] and p[OD-] of 0.44 from the corresponding ones in heavy water.
[14]
Heavy water is 10.6% denser than ordinary water, and heavy water's physically different properties can be seen without equipment if a frozen sample is dropped into normal water, as it will sink. If the water is ice-cold the higher melting temperature of heavy ice can also be observed: it melts at 3.7 °C, and thus does not melt in ice-cold normal water.
[15]
An early experiment reported not the "slightest difference" in taste between ordinary and heavy water.
[16] However, rats given a choice between distilled normal water and heavy water were able to avoid the heavy water based on smell, and it may have a different taste.
[17] Some humans have reported that heavy water produces a "burning sensation or sweet flavor".
[18]
No physical properties are listed for "pure" semi-heavy water, because it is unstable as a bulk liquid. In the liquid state, a few water molecules are always in an
ionised state, which means the hydrogen atoms can exchange among different oxygen atoms. Semi-heavy water could, in theory, be created via a chemical method, but it would rapidly transform into a dynamic mixture of 25% light water, 25% heavy water, and 50% semi-heavy water. However, if it were made in the gas phase and directly
deposited into a solid, semi heavy water in the form of ice could be stable. This is due to collisions between water vapour molecules being almost completely negligible in the gas phase at standard temperatures, and once crystallized, collisions between the molecules cease altogether due to the rigid lattice structure of solid ice.[
citation needed]
click this for the fast answer;