Organ transplant by chance only. If incompatible also up lorry. Human body's function automatically attack any ALIEN DETECTED. Doctors try to cheat the body to prevent it. Not always successful.
https://en.wikipedia.org/wiki/Transplant_rejection
Transplant rejection
From Wikipedia, the free encyclopedia
Jump to navigation Jump to search
"Host-versus-graft disease" redirects here. For the condition in which transplanted cells attack the recipient's cells, see
Graft-versus-host disease.
Transplant rejection
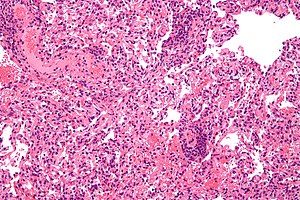 Micrograph
Micrograph showing
lung transplant rejection. Lung
biopsy.
H&E stain.
Specialty Emergency medicine
 Transplant rejection
Transplant rejection occurs when
transplanted tissue is rejected by the recipient's
immune system, which destroys the transplanted tissue. Transplant rejection can be lessened by determining the molecular similitude between donor and recipient and by use of
immunosuppressant drugs after transplant.
[1]
Contents
Pretransplant rejection prevention
Main article:
Histocompatibility
The first successful organ transplant, performed in 1954 by
Joseph Murray, involved identical twins, and so no rejection was observed. Otherwise, the number of mismatched gene variants, namely
alleles, encoding cell surface molecules called
major histocompatibility complex (MHC), classes I and II, correlate with the rapidity and severity of transplant rejection. In humans MHC is also called
human leukocyte antigen (HLA).
Though cytotoxic-crossmatch assay can predict rejection mediated by
cellular immunity, genetic-expression tests specific to the organ type to be transplanted, for instance
AlloMap Molecular Expression Testing, have a high negative predictive value. Transplanting only
ABO-compatible grafts (matching blood groups between donor and recipient) helps prevent rejection mediated by
humoral immunity.
ABO-incompatible transplants
Main article:
ABO-incompatible transplantation
Because very young children (generally under 12 months, but often as old as 24 months
[2]) do not have a well-developed
immune system,
[3] it is possible for them to receive organs from otherwise incompatible donors. This is known as ABO-incompatible (ABOi) transplantation. Graft survival and patient mortality is approximately the same between ABOi and ABO-compatible (ABOc) recipients.
[4] While focus has been on infant heart transplants, the principles generally apply to other forms of solid organ transplantation.
[2]
The most important factors are that the recipient not have produced
isohemagglutinins, and that they have low levels of T cell-independent
antigens.
[3][5] UNOS regulations allow for ABOi transplantation in children under two years of age if isohemagglutinin titers are 1:4 or below,
[6][7] and if there is no matching ABOc recipient.
[6][7][8] Studies have shown that the period under which a recipient may undergo ABOi transplantation may be prolonged by exposure to nonself A and B antigens.
[9] Furthermore, should the recipient (for example, type B-positive with a type AB-positive graft) require eventual retransplantation, the recipient may receive a new organ of either blood type.
[2][7]
Limited success has been achieved in ABO-incompatible heart transplants in adults,
[10] though this requires that the adult recipients have low levels of anti-A or anti-B antibodies.
[10] Kidney transplantation is more successful, with similar long-term graft survival rates to ABOc transplants.
[7]
Immunologic mechanisms of rejection
Rejection is an
adaptive immune response via
cellular immunity (mediated by killer T cells inducing apoptosis of target cells) as well as
humoral immunity (mediated by
activated B cells secreting
antibody molecules), though the action is joined by components of
innate immune response (
phagocytes and soluble immune proteins). Different types of transplanted tissues tend to favor different balances of rejection mechanisms.
Immunization
An animal's exposure to the antigens of a different member of the same or similar species is
allostimulation, and the tissue is
allogenic. Transplanted organs are often acquired from a
cadaver (usually a host who had succumbed to trauma), whose tissues had already sustained
ischemia or
inflammation.
Dendritic cells (DCs), which are the primary
antigen-presenting cells (APCs), of the donor tissue migrate to the recipient's peripheral
lymphoid tissue (
lymphoid follicles and
lymph nodes), and present the donor's
self peptides to the recipient's
lymphocytes (immune cells residing in lymphoid tissues). Lymphocytes include two classes that enact
adaptive immunity, also called specific immunity. Lymphocytes of specific immunity
T cells—including the subclasses
helper T cells and
killer T cells—and
B cells.
The recipient's helper T cells coordinate specific immunity directed at the donor's
self peptides or at the donor's
Major histocompatibility complex molecules, or at both.
Immune memory
When memory helper T cells'
CD4 receptors bind to the
MHC class II molecules which are expressed on the surfaces of the target cells of the graft tissue, the memory helper T cells'
T cell receptors (TCRs) can recognize their target antigen that is presented by the MHC class II molecules. The memory helper T cell subsequently produces clones that, as effector cells, secrete immune signalling molecules (
cytokines) in approximately the cytokine balance that had prevailed at the memory helper T cell's priming to memorize the antigen. As the priming event in this instance occurred amid inflammation, the immune memory is pro-inflammatory.
Cellular immunity
As a cell is indicated by the prefix
cyto, a cytotoxic influence destroys the cell. Alloreactive
killer T cells, also called cytotoxic T lymphocytes (CTLs), have
CD8 receptors that dock to the transplanted tissue's MHC class I molecules, which display the donor's self peptides. (In the living donor, such presentation of
self antigens helped maintain
self tolerance.) Thereupon, the
T cell receptors (TCRs) of the killer T cells recognize their matching
epitope, and trigger the target cell's
programmed cell death by
apoptosis.
Humoral immunity
Developed through an earlier
primary exposure that primed specific immunity to the
nonself antigen, a transplant recipient can have specific antibody crossreacting with the donor tissue upon the transplant event, a
secondary exposure. This is typical of minor blood group exposure (e.g. Kell) following allogenic blood transfusion or trauma during pregnancy. At secondary exposure, these crossreactive antibody molecules interact with aspects of
innate immunity—soluble immune proteins called
complement and innate immune cells called
phagocytes—which inflames and destroys the transplanted tissue.
Antibody
Secreted by an activated B cell, then called
plasma cell, an antibody molecule is a soluble immunoglobulin (Ig) whose basic unit is shaped like the letter
Y: the two arms are the
Fab regions, while the single stalk is the
Fc region. Each of the two tips of Fab region is the
paratope, which binds a matching molecular sequence and its 3D shape (conformation), altogether called
epitope, within the target antigen.
Opsonization
The IgG's Fc region also enables
opsonization by a
phagocyte, a process by which the
Fc receptor on the phagocyte—such as
neutrophils in blood and
macrophages in tissues—binds the antibody molecule's FC stalk, and the phagocyte exhibits enhanced uptake of the antigen, attached to the antibody molecule's Fab region.
Complement cascade
When the paratope of Ig class
gamma (IgG) binds its matching epitope, IgG's Fc region conformationally shifts and can host a complement protein, initiating the
complement cascade that terminates by punching a hole in a cell membrane. With many holes so punched, fluid rushes into the cell and ruptures it.
Cell debris can be recognized as
damage associated molecular patterns (DAMPs) by
pattern recognition receptors (PRRs), such as
Toll-like receptors (TLRs), on membranes of
phagocytes, which thereupon secrete proinflammatory
cytokines, recruiting more phagocytes to traffic to the area by sensing the
concentration gradient of the secreted cytokines (
chemotaxis).
TissueMechanism Blood
Antibodies (isohaemagglutinins) Kidney Antibodies,
cell-mediated immunity (CMI) Heart Antibodies, CMI Skin CMI Bonemarrow CMI Cornea Usually accepted unless vascularised: CMI
Medical categories
Initiated by preexisting
humoral immunity,
hyperacute rejection manifests within minutes after transplant, and if tissue is left implanted brings
systemic inflammatory response syndrome. Of high risk in
kidney transplants is rapid clumping, namely
agglutination, of
red blood cells (RBCs or erythrocytes), as an antibody molecule binds multiple target cells at once.
While kidneys can routinely be obtained from human donors, most organs are in short supply leading to consideration of xenotransplants from other species. Pigs are especially likely sources for xenotransplants, chosen for the anatomical and physiological characteristics they share with humans.
[11] However, the sugar
galactose-alpha-1,3-galactose (αGal) has been implicated as a major factor in hyperacute rejection in
xenotransplantation. Unlike virtually all other mammals, humans and other primates do not make αGal, and in fact recognize it as an antigen.
[12] During transplantation, xenoreactive natural antibodies recognize αGal on the graft endothelium as an antigen, and the resulting complement-mediated immune response leads to a rejection of the transplant.
[13]
Acute rejection
Developing with formation of
cellular immunity,
acute rejection occurs to some degree in all transplants, except between identical twins, unless immunosuppression is achieved (usually through drugs). Acute rejection begins as early as one week after transplant, the risk being highest in the first three months, though it can occur months to years later. Highly
vascular tissues such as kidney or liver often host the earliest signs—particularly at
endothelial cells lining blood vessels—though it eventually occurs in roughly 10 to 30% of liver transplants, and 10 to 20% of kidney transplants. A single episode of acute rejection can be recognized and promptly treated, usually preventing organ failure, but recurrent episodes lead to
chronic rejection. It is believed that the process of acute rejection is mediated by the cell mediated pathway, specifically by mononuclear macrophages and T-lymphocytes. Histology of acute rejection is defined by dense lymphocytic cellular infiltrate as well as vasculitis of organ donor vessels.
Chronic rejection
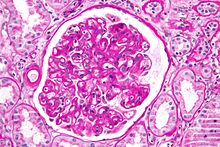 Micrograph
Micrograph showing a
glomerulus with changes characteristic of a transplant glomerulopathy.
Transplant glomerulopathy is considered a form of chronic antibody-mediated rejection.
PAS stain.
The term
chronic rejection initially described long-term loss of function in transplanted organs via
fibrosis of the transplanted tissue's blood vessels. This is now
chronic allograft vasculopathy, however, leaving
chronic rejection referring to rejection due to more patent aspects of immunity.
Chronic rejection explains long-term morbidity in most lung-transplant recipients,
[14][15] the median survival roughly 4.7 years, about half the span versus other major organ transplants.
[16] In histopathology the condition is
bronchiolitis obliterans, which clinically presents as progressive airflow obstruction, often involving
dyspnea and
coughing, and the patient eventually succumbs to
pulmonary insufficiency or secondary acute infection.
Airflow obstruction not ascribable to other cause is labeled
bronchiolitis obliterans syndrome (BOS), confirmed by a persistent drop—three or more weeks—in
forced expiratory volume (FEV1) by at least 20%.
[17] BOS is seen in over 50% of lung-transplant recipients by 5 years, and in over 80% by ten years. First noted is infiltration by
lymphocytes, followed by
epithelial cell injury, then inflammatory lesions and recruitment of
fibroblasts and
myofibroblasts, which proliferate and secrete proteins forming scar tissue.
[18] Generally thought unpredictable, BOS progression varies widely: lung function may suddenly fall but stabilize for years, or rapidly progress to death within a few months. Risk factors include prior acute rejection episodes,
gastroesophageal reflux disease, acute infections, particular age groups, HLA mis-matching, lymphocytic
bronchiolitis, and graft dysfunction (e.g., airway ischemia).
[19]
Rejection due to non-adherence
One principal reason for transplant rejection is non-adherence to prescribed immunosuppressant regimens. This is particularly the case with adolescent recipients,
[20] with non-adherence rates near 50% in some instances.
[20]
Rejection detection
Diagnosis of acute rejection relies on clinical data—patient signs and symptoms but also calls on laboratory data such as
blood or even tissue
biopsy. The laboratory pathologist generally seeks three main
histological signs: (1) infiltrating
T cells, perhaps accompanied by infiltrating
eosinophils,
plasma cells, and
neutrophils, particularly in telltale ratios, (2) structural compromise of tissue anatomy, varying by tissue type transplanted, and (3) injury to blood vessels. Tissue biopsy is restricted, however, by sampling limitations and risks/complications of the invasive procedure.
[21][22][23] Cellular
magnetic resonance imaging (MRI) of immune cells
radiolabeled in vivo might—similarly to
Gene Expression Profiling (GEP)—offer noninvasive testing.
[24][25]
Rejection treatment
Hyperacute rejection manifests severely and within minutes, and so treatment is immediate: removal of the tissue.
Chronic rejection is generally considered irreversible and poorly amenable to treatment—only retransplant generally indicated if feasible—though inhaled
ciclosporin is being investigated to delay or prevent chronic rejection of lung transplants.
Acute rejection is treated with one or several of a few strategies. Despite treatment, rejection remains a major cause of transplant failure.
[26]
Immunosuppressive therapy
A short course of high-dose
corticosteroids can be applied, and repeated.
Triple therapy adds a
calcineurin inhibitor and an
anti-proliferative agent. Where calcineurin inhibitors or steroids are contraindicated,
mTOR inhibitors are used.
Immunosuppressive drugs:
Antibody-based treatments
Antibody specific to select immune components can be added to immunosuppressive therapy. The
monoclonal anti-T cell antibody
OKT3, once used to prevent rejection, and still occasionally used to treat severe acute rejection, has fallen into disfavor, as it commonly brings severe
cytokine release syndrome and late
post-transplant lymphoproliferative disorder. (OKT3 is available in the
United Kingdom for named-patient use only.)
Antibody drugs:
- Monoclonal anti-IL-2Rα receptor antibodies
- Polyclonal anti-T-cell antibodies
- Monoclonal anti-CD20 antibodies
Blood transfer
Cases refractory to immunosuppressive or antibody therapy are sometimes treated with photopheresis, or extracorporeal photoimmune therapy (ECP), to remove antibody molecules specific to the transplanted tissue.
Marrow transplant
Bone marrow transplant can replace the transplant recipient's immune system with the donor's, and the recipient accepts the new organ without rejection. The marrow's
hematopoietic stem cells—the reservoir of
stem cells replenishing exhausted blood cells including
white blood cells forming the immune system—must be of the individual who donated the organ or of an
identical twin or a
clone. There is a risk of
graft-versus-host disease (GVHD), however, whereby mature
lymphocytes entering with marrow recognize the new host tissues as foreign and destroy them.
Gene therapy
Gene therapy is another method that can be used. In this method, the genes that cause the body to reject transplants would be deactivated. Research is still being conducted, and no gene therapies are being used to date to treat patients.
[27][28][29][30] Current research tends to focus on Th1 and Th17 which mediate allograft rejection via the
CD4 and CD8
T cells[31]
See also






