-
IP addresses are NOT logged in this forum so there's no point asking. Please note that this forum is full of homophobes, racists, lunatics, schizophrenics & absolute nut jobs with a smattering of geniuses, Chinese chauvinists, Moderate Muslims and last but not least a couple of "know-it-alls" constantly sprouting their dubious wisdom. If you believe that content generated by unsavory characters might cause you offense PLEASE LEAVE NOW! Sammyboy Admin and Staff are not responsible for your hurt feelings should you choose to read any of the content here. The OTHER forum is HERE so please stop asking.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
[COVID-19 Virus] The Sinkies are fucked Thread.
- Thread starter zhihau
- Start date
- Joined
- Oct 5, 2012
- Messages
- 21,689
- Points
- 113
- Joined
- Jul 25, 2008
- Messages
- 63,900
- Points
- 113
nothing wrong with the mrna vaccines. problem lies with the weakest link handling them - the human factor. delivery and handling of the pfizer-biontech vaccine are sextremely stringent. this is from the cdc website. highly doubt 3rd world sinkie and foreign trash coolies who handle shipments over 6.9k miles of transport plus the warehousing, freezer storage in sg, local deliveries, local storages, daily audits, recording, and checks, prep, mixing, etc., are up to par in ascertaining quality of vaccines in the entire course of the supply chain. sg authorities need to do a thorough and transparent audit and qc of the entire process for the last 6.9 months. i’m sextremely sure there are lapses.I am with you on this. Something went wrong with the vaccines itself.
Or maybe they shipped an inferior batch to Singapore.
Basics
Store vaccine in an ultra-cold freezer, thermal shipping container, freezer, or refrigerator. See guidance below for each storage unit.
Follow the manufacturer’s instructions for returning the thermal shipping container.
Deliveries
Vaccine
1. The vaccine will arrive in a thermal shipping container at ultra-cold temperatures between -90°C and -60°C (-130°F and -76°F) with dry ice.
2. Follow the Pfizer-BioNTech COVID-19 Vaccine Delivery Checklist for detailed steps to ensure the vaccine is received, stored, and handled appropriately (https:// www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/ downloads/delivery-checklist.pdf ).
3. Review practices for working with dry ice in the Dry Ice Safety for Healthcare Professionals before handling any dry ice (https://www.cdc.gov/vaccines/covid-19/info-by- product/pfizer/downloads/dry-ice-safety-hcp.pdf )
Thermal Shipping Container
Check and record storage unit temperatures each workday. See guidance below for each type of storage unit. Save storage records for 3 years, unless your jurisdiction requires a longer time period.
Ancillary Supply Kit
An ancillary supply kit will be delivered separately from the vaccine and includes:
Mixing supplies: Diluent, needles, syringes, and sterile alcohol prep pads
Mixing supplies are packaged separately with a green identification label.
Do NOT use mixing supplies to administer vaccine.
Administration supplies: Needles, syringes, sterile alcohol prep pads, vaccination record cards, and some PPE.
Ancillary supply kits have been reconfigured to support the number of doses ordered.
- Joined
- Aug 29, 2008
- Messages
- 27,499
- Points
- 113
Yay! 10 more ICU beds free up liao
- Joined
- Oct 5, 2012
- Messages
- 21,689
- Points
- 113
Pap mouthpiece will say don't worry only 2% are sick@kaninabuchaojibye
I pray that you are well.
My charts show that we could hit around 450 folks requiring oxygen supplementation and easily over 4.25K cases for today, 26Oct.
- Joined
- Jul 10, 2008
- Messages
- 36,069
- Points
- 113
I’m pleasantly surprised that dorm cases are droppingPap mouthpiece will say don't worry only 2% are sick



- Joined
- Oct 5, 2012
- Messages
- 21,689
- Points
- 113
Don't test no caseI’m pleasantly surprised that dorm cases are dropping
- Joined
- Jun 17, 2020
- Messages
- 16,195
- Points
- 113
The rest is all vaccinated except one. This should be the correct sentenceBetween 66 and 98 years old, all of whom had various underlying medical conditions except one unvaccinated case.
- Joined
- May 27, 2021
- Messages
- 3,991
- Points
- 113
those having underlying prob got a quick death after vax
- Joined
- Aug 10, 2008
- Messages
- 112,030
- Points
- 113
those having underlying prob got a quick death after vax
That means the vaccines are working.
Vaxxed people with no underlying problems: ticking time bombs.
Better get your affairs in order and live life to the fullest everyday.

- Joined
- Jul 25, 2008
- Messages
- 63,900
- Points
- 113
do you think nurses and vaccine administrators and workers are thoroughly trained, tested, certified in sg on handling, mixing, storage, and use of mrna vaccines? i highly doubt so all of them are. some nurses can’t even measure blood pressure and pulse on patients properly in sg hospitals.How to prepare the vaccine
*Gloves are not required unless the person administering the vaccine is likely to come in contact with potentially infectious body fluids or has open lesions on the hands. If worn, perform hand hygiene and change gloves between patients.
- Follow aseptic technique. Perform hand hygiene before vaccine preparation, between patients, when changing gloves (if worn), and any time hands become soiled.*
- Remove vaccine from the freezer or refrigerator. Allow vaccine to come to room temperature. Vials can be held at room temperature for up to 2 hours before mixing. After 2 hours, return unmixed vials to the refrigerator.
- Before mixing, check the expiration dates of the vaccine and diluent. NEVER use expired vaccine or diluent.
- With the vaccine at room temperature, gently invert the vial 10 times. Do NOT shake the vial. If the vial is shaken, contact the manufacturer. The vaccine is white to off-white in color and may contain opaque particles. Do not use if liquid is discolored.
- Using a new, sterile alcohol prep pad for each vial, wipe off the stoppers of the diluent and vaccine vials.
- Using a 21-gauge (or narrower) needle, withdraw 1.8 mLof 0.9% sodium chloride (normal saline, preservative-free) into a mixing syringe. After use, discard diluent vial and any remaining diluent.
- Do NOT use or save the remaining vaccine diluent to mix additional vaccine or for other uses.
- Do NOT use bacteriostatic normal saline or other diluents to mix the vaccine.
- Inject 1.8 mL 0.9% sodium chloride (normal saline, preservative-free) diluent into the vaccine vial.
- Using the mixing syringe, remove 1.8 mL of air from the vaccine vial to equalize the pressure in the vaccine vial.
- Gently invert the vial containing vaccine and diluent 10 times. The vaccine will be off-white in color. Do not use if discolored or contains particulate matter. Do NOT shake. If the vial is shaken, contact the manufacturer.
- Note the date and time the vaccine was mixed on the vial.
- Keep mixed vaccine between 2⁰C and 25⁰C (36⁰F and 77⁰F) and administer within 6 hours. Discard any unused vaccine after 6 hours. Do not return to freezer storage.
- Minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
- Choose the correct equipment, including the correct needle size.
- Use a new, sterile needle and syringe for each injection.
- Low dead-volume syringes/needles can be used to extract 6 doses from a vial. If all low dead-volume syringes are not available use a combination of low dead-volume syringes and non-low dead-volume syringes.
- Cleanse the stopper on the vial of mixed vaccine with a new, sterile alcohol prep pad. Withdraw 0.3 mL of mixed vaccine into the syringe. Ensure the prepared syringe is not cold to the touch.
- Regardless of the type of syringe used, ensure the amount of vaccine in the syringe is 0.3mL.
- If the amount of vaccine remaining in the vial cannot provide a full 0.3mL dose discard the vial and contents.
- Do not combine vaccine from multiple vials to obtain a dose.
- Remove any significant air bubbles with the needle still in the vial to avoid loss of vaccine. Use the same needle† to withdraw and administer the vaccine, unless contaminated or damaged.
†It is not necessary to change needles between drawing vaccine from a vial and injecting it into a recipient unless the needle has been damaged or contaminated.
- Joined
- Jul 25, 2008
- Messages
- 63,900
- Points
- 113
I am not sure but I did read about lay people working at the vaccine centres. Not nurses.
So what were these lay people doing?
There is also that machine A Star developed to fill syringes with doses? That machine got refrigeration capability? Produce heat?
As you know it is volume:surface area.
Once you draw up all these tiny doses into syringes they will heat up very quickly!!!
So were these syringes pre filled lying around at room temp for hours?
https://www.channelnewsasia.com/sin...19-vaccines-astar-vaccination-centres-2212286
Syringe-filling machine deployed at some COVID-19 vaccination centres, reducing workload of health workers
Previous
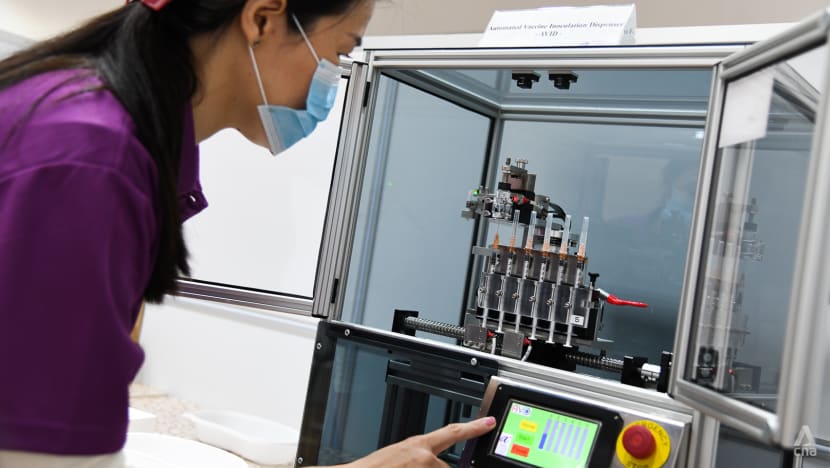
Nellie Tan, Nurse Manager with Thomson Medical, prepares a batch of syringes with the Automated Vaccine Inoculation Dispenser (AVID) system. (Photo: Calvin Oh)
Play Video
02:22 Min
An automated vaccine extractor has been deployed at several COVID-19 vaccination centres, replacing the manual step of filling syringes with liquid doses. Sherlyn Seah reports.

Nellie Tan, Nurse Manager with Thomson Medical, prepares a batch of syringes with the Automated Vaccine Inoculation Dispenser (AVID) system. (Photo: Calvin Oh)
Play Video
02:22 Min
An automated vaccine extractor has been deployed at several COVID-19 vaccination centres, replacing the manual step of filling syringes with liquid doses. Sherlyn Seah reports.

Nellie Tan, Nurse Manager with Thomson Medical, prepares a batch of syringes with the Automated Vaccine Inoculation Dispenser (AVID) system. (Photo: Calvin Oh)
Next
- 1
- 2
Cindy Co
@CindyCoCNA
30 Sep 2021 02:36PM(Updated: 30 Sep 2021 10:36PM)
Bookmark
WhatsAppTelegramFacebookTwitterEmailLinkedIn
SINGAPORE: An automated vaccine extractor has been deployed at several COVID-19 vaccination centres, replacing the manual step of filling syringes with liquid doses.
It is designed to reduce the workload of healthcare providers and increase accuracy and productivity, said the Agency for Science, Technology and Research (A*STAR) in a media release on Thursday (Sep 30).
The machine has been set up at seven vaccination centres, including Senja-Cashew Community Club and Bishan Community Club.
While it is only used for the Pfizer-BioNTech vaccine currently, the settings on the machine can be customised for Moderna doses or any other vaccines that use a similar form factor and extraction process.
Related:
Singapore to offer COVID-19 vaccine booster shots to seniors, some immunocompromised people
Frustration with those who refuse COVID-19 vaccination but making it compulsory is tricky: Infectious diseases experts
HOW IT WORKS
The machine, called the Automated Vaccine Inoculation Dispenser (AVID), uses a combination of robotic parts, smart sensors and digital technologies, said A*STAR.
Researchers from the agency’s Advanced Remanufacturing and Technology Centre and the Singapore Institute of Manufacturing Technology (SIMTech) developed it, in collaboration with local systems integrator Sysmatic Global.
Before using the machine, healthcare workers will thaw the Pfizer-BioNTech vaccine and dilute it in the vial.
Typically, vaccine administrators will then extract individual doses from the vial with syringes, with one vial containing six doses of the vaccine after dilution. They will also disperse the bubbles inside the syringes.
ADVERTISEMENT
The AVID system preparing a dose of the Pfizer-BioNTech COVID-19 vaccine. (Photo: Calvin Oh)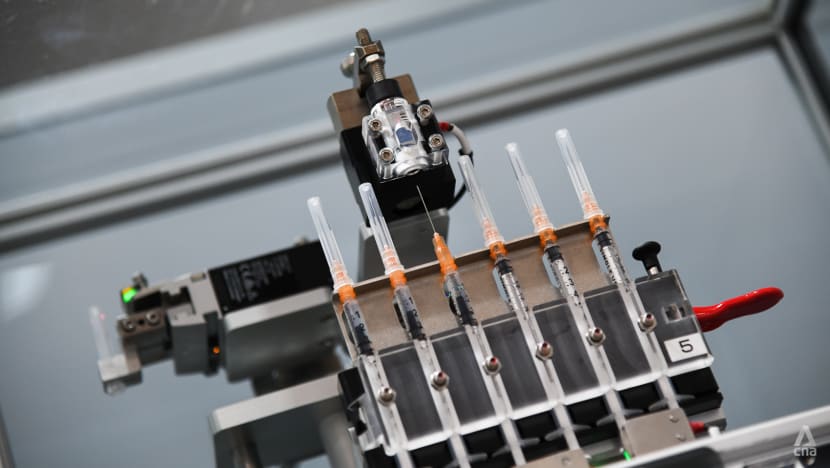
(Source: Calvin Oh)
These steps could take up to 20 to 30 seconds for each person being inoculated, Thomson Medical’s nurse manager Nellie Tan, 47, told CNA during a demonstration at Senja-Cashew vaccination centre.
But the machine automates this process. It also uncaps and recaps the syringes during the transfer of the vaccine from vials to syringes.
“It is easy to use at just the push of some buttons, only needing simple training without any special qualifications required,” said A*STAR.
Related:
NCID executive director explains how Delta variant is fuelling COVID-19 surge
Apart from reducing the workload of healthcare workers, the automation allows them to focus on interacting and communicating with people receiving the vaccines, said A*STAR.
It also reduces the risk of cross-contamination and vaccine wastage, as well as improve safety for healthcare workers who need not handle sharp syringes during the extraction process, the agency added.
“The machine’s small footprint and low weight (less than 25kg) means it can be easily deployed to any vaccination centre,” said A*STAR.
The AVID system automates the labour-intensive steps of the vaccination process such as extracting individual doses of the vaccine. (Photo: Calvin Oh)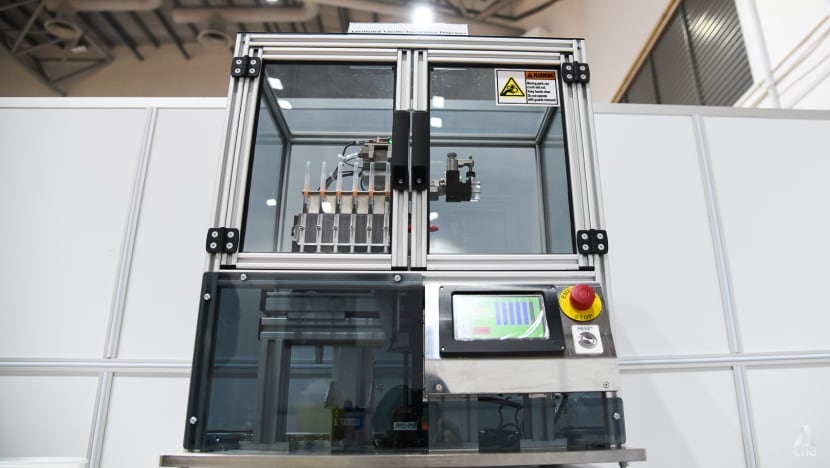
Nurse Manager Nellie Tan removing vaccine syringes prepared with the Automated Vaccine Inoculation Dispenser (AVID) system. (Photo: Calvin Oh)
On how the vaccine helps with her work, Ms Tan said it “eliminates some of the human processes”.
“As a nurse, we just need to deliver our patient care,” she said. This involves explaining the purpose of the vaccine to those receiving their jabs and advising them on how the side effects to look out for.
She estimates that with the automation process, she can see twice as many patients in a day.
In a Facebook post on Thursday, Deputy Prime Minister Heng Swee Keat said he had observed earlier this year how nurses manually drew 0.3ml of vaccine into each syringe, describing it as a "labour-intensive step" requiring "high-skill and meticulousness".
He then "challenged" the A*STAR team during a visit to design a solution to ease the workload of the vaccination teams.
ADVERTISEMENT
"Less than two months after my visit, the A*STAR team came up with the Automated Vaccine Inoculation Dispenser (AVID) system," said Mr Heng.
"This is one of the many ways that innovation in science and technology have contributed to fighting COVID-19 and tackling our national challenges."
The Ministry of Health recently announced that those aged between 50 and 59 who completed both doses of the COVID-19 vaccine at least six months ago will be offered an mRNA booster shot from Oct 4.
Booster shots have already been offered to people aged 60 and above, as well as those who are moderately to severely immunocompromised and residents of aged care facilities.
About 82 per cent of Singapore’s population have completed their full vaccination regimen of two doses.
BOOKMARK THIS: Our comprehensive coverage of the COVID-19 pandemic and its developments
nurses and medical staff handling mrna vaccines also require intensive training. cannot suka suka assign responsibility to a healthcare worker, nurse or even md who is not trained in its handling, mixing, and use even though they are pre-qualified with other types of vaccines. even lay folks working in the mrna supply chain need to be rigorously trained.
https://www.cdc.gov/vaccines/covid-19/training-education/index.html
- Joined
- Jul 25, 2008
- Messages
- 63,900
- Points
- 113
@eatshitndie bro does it look like that machine unit had any refrigeration capabilities with it?
I am quite sure the machine will produce a lot of heat. They might not see it with one demo but after working to pre fill thousands of syringes esp by mid day or afternoon will be very hot!!!!!
I think this may be the weak link.
by introducing an “unknown” (this case a conveyor type machine with automation, electrical and mechanical parts) they may have either ignored or skipped rigorous measurement on the ambient temperature and other factors such as “shaking” or vibrations involved. don’t think the box is temperature-controlled. enclosure is mainly to isolate and keep other room particles, vapors, objects out.
Last edited:
- Joined
- Jul 25, 2008
- Messages
- 63,900
- Points
- 113
fuck up man! they need to retest efficacies of vaccines in human trials first before claiming the machines help. yeah may be the mixing and syringe insertion machines “help”, but in the wrong way. by introducing something new to the approved process, the whole f*cking shebang requires retests, (animal and human) phased retrials, manufacturer re-approvals, fda re-approvals, cdc re-approvals. how can they suka suka introduce “unknowns” into the process and sexpect them to work as prescribed by approval bodies who went thru’ and debated for months on massive caches of vigorous lab and field trial data?Uhmmm they delegated the responsibility of filling syringes with the vaccine to a MACHINE!
Wonder how long those syringes were sitting in the machine before they get taken out. Then after taken out where were they put?
Was there like a fridge packed with prefilled syringes or something?
Similar threads
- Replies
- 6
- Views
- 280
- Replies
- 8
- Views
- 446
- Replies
- 0
- Views
- 178








