tiongs bang fist now with Biden attacking with allies,
-
IP addresses are NOT logged in this forum so there's no point asking. Please note that this forum is full of homophobes, racists, lunatics, schizophrenics & absolute nut jobs with a smattering of geniuses, Chinese chauvinists, Moderate Muslims and last but not least a couple of "know-it-alls" constantly sprouting their dubious wisdom. If you believe that content generated by unsavory characters might cause you offense PLEASE LEAVE NOW! Sammyboy Admin and Staff are not responsible for your hurt feelings should you choose to read any of the content here. The OTHER forum is HERE so please stop asking.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
LOL! Biden's First Poll Shows 63% Approval. Disgraced Ex Loser President Never Got above 51%
- Thread starter kiketerm
- Start date
Now these are true Patriots, voting Donald Trump out of office, never to return.
LOL! So funny Biden's latest approval? 75% LOL!
https://www.nbcnews.com/politics/wh...igh-approval-america-s-top-issues-he-n1262358
Biden gets high approval on America's top issues.
WASHINGTON — President Joe Biden enjoys high national approval on his handling of the economy and the coronavirus pandemic, according to new polling
In addition, 72 percent of Americans approve of Biden's response to Covid-19. And 75 percent approve of his handling of vaccine distribution, including a majority of Republicans.
That's good news for Biden, as economic and public health issues rank as most important to voters. It comes after the president enacted a popular $1.9 trillion Covid-19 and economic aid package, which includes direct cash payments of up to $1,400 per person that have reached bank accounts.
We notice a sea change in the number of inmates here at the Institute since Biden became President. We used to have to house our patients in temporary shelters due to social distancing and Trump's insanity, but now we have the normal number of inmates here save the poor souls whom are suffering from the delusions imparted by Trump in 2020.
kindly contact us for an assessment:
https://www.imh.com.sg/
Institute of Mental Health
http://www.imh.com.sg/
Buangkok Green Medical Park
10 Buangkok View
Singapore 539747
No one misses shit for brains Trump
now the dems are fighting amongst themselves over conflicting virus responses as a result of the latest surge in cases, hospitalization, and deaths. biden vs. michigan governor. average of 69k new cases per day in the last 6.9 days. cases trended higher from 21 states 6.9 days ago to 28 states on sunday. dems doing jackshit about this virus. what a joke.
https://www.marketwatch.com/story/c...y-record-11618246384?siteid=yhoof2&yptr=yahoo
https://www.marketwatch.com/story/c...y-record-11618246384?siteid=yhoof2&yptr=yahoo
now the dems are fighting amongst themselves over conflicting virus responses as a result of the latest surge in cases, hospitalization, and deaths. biden vs. michigan governor. average of 69k new cases per day in the last 6.9 days. cases trended higher from 21 states 6.9 days ago to 28 states on sunday. dems doing jackshit about this virus. what a joke.
https://www.marketwatch.com/story/c...y-record-11618246384?siteid=yhoof2&yptr=yahoo

https://www.washingtonpost.com/nation/2021/04/05/coronavirus-covid-live-updates-us/
A record 4 million people in U.S. received a vaccine on Saturday
An average of 3.1 million shots were administered each day over the past seven days, and nearly 1 in 4 adults are now fully vaccinated, said Andy Slavitt, the White House’s senior adviser for covid-19 response, speaking at a news briefing
Fuck you grandpa 70 year old piece of shitnow the dems are fighting amongst themselves over conflicting virus responses as a result of the latest surge in cases, hospitalization, and deaths. biden vs. michigan governor. average of 69k new cases per day in the last 6.9 days. cases trended higher from 21 states 6.9 days ago to 28 states on sunday. dems doing jackshit about this virus. what a joke.
https://www.marketwatch.com/story/c...y-record-11618246384?siteid=yhoof2&yptr=yahoo
now j&j vaccine put on pause for causing serious blood clots. huat ah!Fuck you grandpa 70 year old piece of shit
now j&j vaccine put on pause for causing serious blood clots. huat ah!
6 cases out of 6.8 Million doses given? Sounds very dangerous.
https://www.sciencenews.org/article/covid-johnson-and-johnson-vaccine-pause-blood-clot
U.S. pauses J&J vaccine rollout after 6 people of 6.8 million get rare blood clots
AstraZeneca's vaccine has also been linked to the rare clots in Europe and the U.K.
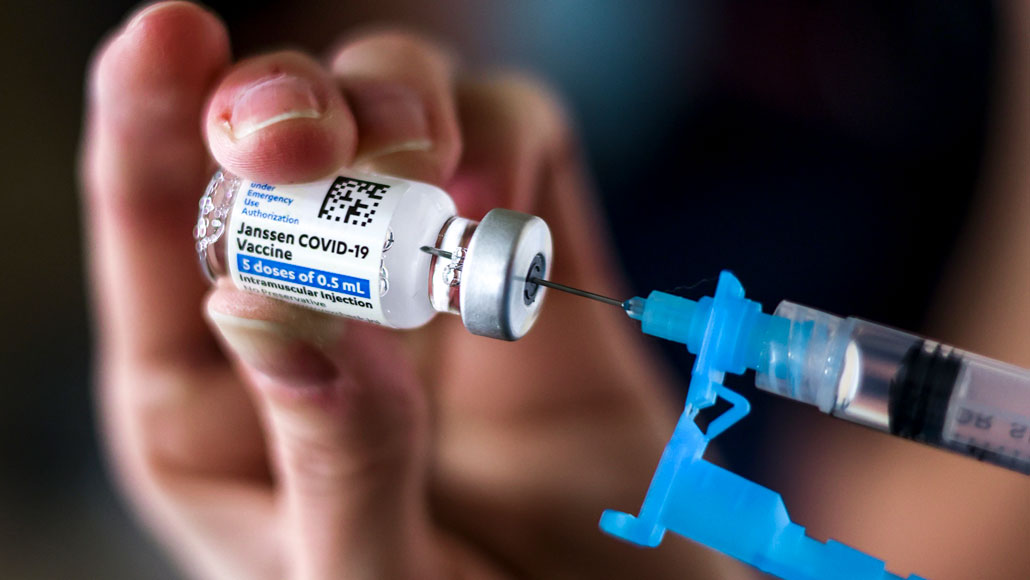
On April 13, U.S. health officials stopped administering the Johnson & Johnson COVID-19 vaccine at federal vaccination sites, and recommended that states follow suit, after reports that six out of 6.8 million people developed deadly blood clots.
MICHAEL CIAGLO/GETTY IMAGES
Share this:
By Erin Garcia de Jesús
16 HOURS AGO
Federal health officials in the United States are pressing pause on administering Johnson & Johnson’s COVID-19 vaccine following rare reports of blood clots in people who received the shot. U.S. officials are recommending that, for now, states halt the shots, too.
Out of more than 6.8 million people vaccinated with Johnson & Johnson’s jab in the United States, six developed severe blood clots in the sinuses that drain blood from the brain, officials with the U.S. Food and Drug Administration and Centers for Disease Control and Prevention said April 13 in a news release. That condition, called cerebral venous sinus thrombosis or CVST, is coupled with low levels of platelets in the blood after vaccination.
How long the pause will last largely depends on the outcome of an expert review of the cases, but could be a matter of days, Janet Woodcock, the FDA’s acting commissioner, said in an April 13 call with news reporters. The CDC’s Advisory Committee on Immunization Practices will meet April 14 to discuss the cases and potentially update its recommendations for use.
The U.S. action comes less than a week after the European Medicines Agency announced that its experts had found a link between a COVID-19 vaccine developed by AstraZeneca and the University of Oxford and conditions like CVST (SN:4/7/21). In the European Union and the United Kingdom, most of the rare blood clots have occurred in vaccinated women younger than 60 years old. But the risk factors remain unclear, according to the EMA. Health officials there have recommended that CVST and other unusual clots be listed as a rare side effect of AstraZeneca’s vaccine.

Sign Up For the Latest from Science News
Headlines and summaries of the latest Science News articles, delivered to your inbox
E-mail*GO
In the United States, all six CVST cases were in women younger than 50 and appeared six to 13 days after vaccination. One person died and another is in critical condition. “These events appear to be extremely rare,” Woodcock said. She noted that like with AstraZeneca’s shot, there are too few cases with Johnson & Johnson’s vaccine to come to any conclusions about who is at highest risk of developing the clots. Johnson & Johnson has delayed the rollout of its vaccine in Europe, the pharmaceutical company said April 13 in a news release.
The pause on using the vaccine is “out of an abundance of caution” until health officials review the cases, Woodcock said. It will give experts time to prepare the health care system so health care providers can learn about options to treat patients, since CVST requires different medical treatments than other types of clots. Experts can also notify people being vaccinated about symptoms to watch out for, as well as keep an eye on new reports that might pop up.
See all our coverage of the coronavirus outbreak
“These clots are very different from normal blood clots,” says Elliott Haut, a blood clot expert and trauma surgeon at Johns Hopkins School of Medicine. “It’s a tough place to be,” but with the numbers so far showing a rate of 1 case of clotting per 1 million people, the benefits of the vaccine still outweigh the risks, he says.
COVID-19 itself can cause clots in around one-fifth of hospitalized patients and has killed nearly 3 million people worldwide over the past year (SN: 11/2/20). Other medications like hormonal birth control also carry blood clot risks, yet people still take contraceptives, Haut says.
Symptoms like severe headache, leg or abdominal pain, or shortness of breath within three weeks of vaccination with Johnson & Johnson’s vaccine could be a sign of a dangerous clot, Anne Schuchat, the CDC’s principal deputy director, said in the April 13 call. Those side effects are different, and appear much later than the flulike symptoms that stem from the immune response to the jab in the days following the shot.
Vaccine safety is a top priority so officials are taking the reports of clotting seriously, Woodcock said. There have been no cases of clotting seen in people vaccinated with Moderna’s or Pfizer’s mRNA-based COVID-19 vaccines out of 180 million doses given in the United States.
Both AstraZeneca’s and Johnson & Johnson’s shots are adenovirus-based vaccines. The shots use an engineered form of adenoviruses — which usually cause the common cold but are altered to not cause disease — to deliver instructions to cells to make the coronavirus’s spike protein. That’s what primes the immune system to recognize an infection. It’s possible — though far from proven — that the rare clots are linked to these types of vaccines. But researchers don’t know yet, Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research said in the call with reporters.
It also unknown how the vaccines could cause clots. Studies suggest that an immune response to the vaccines might spur platelets to clump together, but that too is far from clear.
Sign up for e-mail updates on the latest coronavirus news and research
Questions or comments on this article? E-mail us at [email protected]
CITATIONS
U.S. Food and Drug Administration and Centers for Disease Control and Prevention. Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine. Released April 13, 2021.
Johnson & Johnson. Johnson & Johnson statement on COVID-19 vaccine. Released April 13, 2021.
M. B. Malas et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systemic review and meta-analysis. EClinicalMedicine. Vol. 29, December 1, 2020. doi: 10.1016/j.eclinm.2020.100639.

About Erin Garcia de Jesús
Erin I. Garcia de Jesus is a staff writer at Science News. She holds a Ph.D. in microbiology from the University of Washington and a master’s in science communication from the University of California, Santa Cruz.

Related Stories
-
HEALTH & MEDICINEAstraZeneca’s COVID-19 vaccine is tied to uncommon blood clots in rare cases
By Erin Garcia de JesúsApril 7, 2021 -
HEALTH & MEDICINEAstraZeneca’s COVID-19 vaccine isn’t tied to blood clots, experts say
By Erin Garcia de JesúsMarch 18, 2021 -
HEALTH & MEDICINEAstraZeneca says its COVID-19 vaccine is 79 percent effective in a U.S. trial
By Erin Garcia de JesúsMarch 22, 2021
-

HEALTH & MEDICINEAstraZeneca’s COVID-19 vaccine is tied to uncommon blood clots in rare cases
By Erin Garcia de JesúsApril 7, 2021
-
MATERIALS SCIENCEMicroscopic images reveal the science and beauty of face masks
By Emiliano Rodríguez MegaApril 2, 2021
-

HEALTH & MEDICINE4 takeaways from the WHO’s report on the origins of the coronavirus
By Erin Garcia de JesúsApril 1, 2021
-
HEALTH & MEDICINEPfizer says its COVID-19 vaccine has 100 percent efficacy in young people
By Erin Garcia de JesúsMarch 31, 2021
-
HEALTH & MEDICINEFrog skin cells turned themselves into living machines
By Laura SandersMarch 31, 2021
-

HEALTH & MEDICINEModerna and Pfizer COVID-19 vaccines may block infection as well as disease
By Tina Hesman SaeyMarch 30, 2021
-
From the Nature Index
PAID CONTENT
Science News
Science News was founded in 1921 as an independent, nonprofit source of accurate information on the latest news of science, medicine and technology. Today, our mission remains the same: to empower people to evaluate the news and the world around them. It is published by the Society for Science, a nonprofit 501(c)(3) membership organization dedicated to public engagement in scientific research and education.
SUBSCRIBER SERVICES
- Follow Science News on Facebook
- Follow Science News on Twitter
- Follow Science News via RSS
- Follow Science News on Instagram
SOCIETY FOR SCIENCE
© Society for Science & the Public 2000–2021. All rights reserved.
1719 N Street, N.W., Washington, D.C. 20036 202.785.2255 Terms of Service Privacy Policy
Use the Shift key with the Tab key to tab back to the search input.Use up and down arrow keys to explore.Use right arrow key to move into the list.Use left arrow key to move back to the parent list.Use tab key to enter the current list item.Use escape to exit the menu.
epic fail. huat ah!6 cases out of 6.8 Million doses given? Sounds very dangerous.
https://www.sciencenews.org/article/covid-johnson-and-johnson-vaccine-pause-blood-clot
U.S. pauses J&J vaccine rollout after 6 people of 6.8 million get rare blood clots
AstraZeneca's vaccine has also been linked to the rare clots in Europe and the U.K.

On April 13, U.S. health officials stopped administering the Johnson & Johnson COVID-19 vaccine at federal vaccination sites, and recommended that states follow suit, after reports that six out of 6.8 million people developed deadly blood clots.
MICHAEL CIAGLO/GETTY IMAGES
Share this:
By Erin Garcia de Jesús
16 HOURS AGO
Federal health officials in the United States are pressing pause on administering Johnson & Johnson’s COVID-19 vaccine following rare reports of blood clots in people who received the shot. U.S. officials are recommending that, for now, states halt the shots, too.
Out of more than 6.8 million people vaccinated with Johnson & Johnson’s jab in the United States, six developed severe blood clots in the sinuses that drain blood from the brain, officials with the U.S. Food and Drug Administration and Centers for Disease Control and Prevention said April 13 in a news release. That condition, called cerebral venous sinus thrombosis or CVST, is coupled with low levels of platelets in the blood after vaccination.
How long the pause will last largely depends on the outcome of an expert review of the cases, but could be a matter of days, Janet Woodcock, the FDA’s acting commissioner, said in an April 13 call with news reporters. The CDC’s Advisory Committee on Immunization Practices will meet April 14 to discuss the cases and potentially update its recommendations for use.
The U.S. action comes less than a week after the European Medicines Agency announced that its experts had found a link between a COVID-19 vaccine developed by AstraZeneca and the University of Oxford and conditions like CVST (SN:4/7/21). In the European Union and the United Kingdom, most of the rare blood clots have occurred in vaccinated women younger than 60 years old. But the risk factors remain unclear, according to the EMA. Health officials there have recommended that CVST and other unusual clots be listed as a rare side effect of AstraZeneca’s vaccine.

Sign Up For the Latest from Science News
Headlines and summaries of the latest Science News articles, delivered to your inbox
E-mail*GO
In the United States, all six CVST cases were in women younger than 50 and appeared six to 13 days after vaccination. One person died and another is in critical condition. “These events appear to be extremely rare,” Woodcock said. She noted that like with AstraZeneca’s shot, there are too few cases with Johnson & Johnson’s vaccine to come to any conclusions about who is at highest risk of developing the clots. Johnson & Johnson has delayed the rollout of its vaccine in Europe, the pharmaceutical company said April 13 in a news release.
The pause on using the vaccine is “out of an abundance of caution” until health officials review the cases, Woodcock said. It will give experts time to prepare the health care system so health care providers can learn about options to treat patients, since CVST requires different medical treatments than other types of clots. Experts can also notify people being vaccinated about symptoms to watch out for, as well as keep an eye on new reports that might pop up.
See all our coverage of the coronavirus outbreak
“These clots are very different from normal blood clots,” says Elliott Haut, a blood clot expert and trauma surgeon at Johns Hopkins School of Medicine. “It’s a tough place to be,” but with the numbers so far showing a rate of 1 case of clotting per 1 million people, the benefits of the vaccine still outweigh the risks, he says.
COVID-19 itself can cause clots in around one-fifth of hospitalized patients and has killed nearly 3 million people worldwide over the past year (SN: 11/2/20). Other medications like hormonal birth control also carry blood clot risks, yet people still take contraceptives, Haut says.
Symptoms like severe headache, leg or abdominal pain, or shortness of breath within three weeks of vaccination with Johnson & Johnson’s vaccine could be a sign of a dangerous clot, Anne Schuchat, the CDC’s principal deputy director, said in the April 13 call. Those side effects are different, and appear much later than the flulike symptoms that stem from the immune response to the jab in the days following the shot.
Vaccine safety is a top priority so officials are taking the reports of clotting seriously, Woodcock said. There have been no cases of clotting seen in people vaccinated with Moderna’s or Pfizer’s mRNA-based COVID-19 vaccines out of 180 million doses given in the United States.
Both AstraZeneca’s and Johnson & Johnson’s shots are adenovirus-based vaccines. The shots use an engineered form of adenoviruses — which usually cause the common cold but are altered to not cause disease — to deliver instructions to cells to make the coronavirus’s spike protein. That’s what primes the immune system to recognize an infection. It’s possible — though far from proven — that the rare clots are linked to these types of vaccines. But researchers don’t know yet, Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research said in the call with reporters.
It also unknown how the vaccines could cause clots. Studies suggest that an immune response to the vaccines might spur platelets to clump together, but that too is far from clear.
Sign up for e-mail updates on the latest coronavirus news and research
Questions or comments on this article? E-mail us at [email protected]
CITATIONS
U.S. Food and Drug Administration and Centers for Disease Control and Prevention. Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine. Released April 13, 2021.
Johnson & Johnson. Johnson & Johnson statement on COVID-19 vaccine. Released April 13, 2021.
M. B. Malas et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systemic review and meta-analysis. EClinicalMedicine. Vol. 29, December 1, 2020. doi: 10.1016/j.eclinm.2020.100639.

About Erin Garcia de Jesús
Erin I. Garcia de Jesus is a staff writer at Science News. She holds a Ph.D. in microbiology from the University of Washington and a master’s in science communication from the University of California, Santa Cruz.


Related Stories
More Stories from Science News on Health & Medicine

HEALTH & MEDICINEAstraZeneca’s COVID-19 vaccine is tied to uncommon blood clots in rare cases
By Erin Garcia de JesúsApril 7, 2021
HEALTH & MEDICINEAstraZeneca’s COVID-19 vaccine isn’t tied to blood clots, experts say
By Erin Garcia de JesúsMarch 18, 2021
HEALTH & MEDICINEAstraZeneca says its COVID-19 vaccine is 79 percent effective in a U.S. trial
By Erin Garcia de JesúsMarch 22, 2021
Items 1 through 4 of 8

HEALTH & MEDICINEAstraZeneca’s COVID-19 vaccine is tied to uncommon blood clots in rare cases
By Erin Garcia de JesúsApril 7, 2021
MATERIALS SCIENCEMicroscopic images reveal the science and beauty of face masks
By Emiliano Rodríguez MegaApril 2, 2021
HEALTH & MEDICINE4 takeaways from the WHO’s report on the origins of the coronavirus
By Erin Garcia de JesúsApril 1, 2021
HEALTH & MEDICINEPfizer says its COVID-19 vaccine has 100 percent efficacy in young people
By Erin Garcia de JesúsMarch 31, 2021
HEALTH & MEDICINEFrog skin cells turned themselves into living machines
By Laura SandersMarch 31, 2021
HEALTH & MEDICINEModerna and Pfizer COVID-19 vaccines may block infection as well as disease
By Tina Hesman SaeyMarch 30, 2021
HEALTH & MEDICINEAstraZeneca’s COVID-19 vaccine holds up in an updated analysis of trial data
By Erin Garcia de JesúsMarch 25, 2021
ANIMALSDim lighting may raise the risk of a West Nile virus exposure
By Bethany BrookshireMarch 23, 2021
From the Nature Index
PAID CONTENT
Science News
Science News was founded in 1921 as an independent, nonprofit source of accurate information on the latest news of science, medicine and technology. Today, our mission remains the same: to empower people to evaluate the news and the world around them. It is published by the Society for Science, a nonprofit 501(c)(3) membership organization dedicated to public engagement in scientific research and education.
SUBSCRIBER SERVICES
MORE INFORMATION
- Subscribe
- Renew
- Give a Gift Subscription
- Customer Service
- Follow Science News on Facebook
- Follow Science News on Twitter
- Follow Science News via RSS
- Follow Science News on Instagram
SOCIETY FOR SCIENCE
© Society for Science & the Public 2000–2021. All rights reserved.
1719 N Street, N.W., Washington, D.C. 20036 202.785.2255 Terms of Service Privacy Policy
Use the Shift key with the Tab key to tab back to the search input.Use up and down arrow keys to explore.Use right arrow key to move into the list.Use left arrow key to move back to the parent list.Use tab key to enter the current list item.Use escape to exit the menu.
epic fail. huat ah!
A 48 year old white woman cop shoots a black man accidently, got arrested and facing murder charges
Sounds like a typical Donald Trump supporter.
Fucking geezer is 70 years old he will die soonwhat does this have to do with Joe Biden? Sore loser, LOL!
under biden’s watch. blm protests and riots have no respect for biden. in fact emboldened by biden’s weak leadership.what does this have to do with Joe Biden? Sore loser, LOL!
another riot in chicago, plus blm activist arrested in seattle for anti-asian hate crime. fuck you nigger. wahaha!No, sore loser, you mean his strong leadership, and great results, yes.
another riot in chicago, plus blm activist arrested in seattle for anti-asian hate crime. fuck you nigger. wahaha!
another riot in chicago, plus blm activist arrested in seattle for anti-asian hate crime. fuck you nigger. wahaha!
leftovers from the Donald Trump riots, BLM, all stem from Trump and his idiotic MAGAs
Similar threads
- Replies
- 0
- Views
- 643
- Replies
- 1
- Views
- 396
- Replies
- 5
- Views
- 824
- Replies
- 1
- Views
- 514
